Recent Advances in Rydberg Physics Using Two-Electron Atoms Abstract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calculation of Rydberg Interaction Potentials
Tutorial Calculation of Rydberg interaction potentials Sebastian Weber,1, ∗ Christoph Tresp,2, 3 Henri Menke,4 Alban Urvoy,2, 5 Ofer Firstenberg,6 Hans Peter B¨uchler,1 and Sebastian Hofferberth2, 3, † 1Institute for Theoretical Physics III and Center for Integrated Quantum Science and Technology, Universit¨atStuttgart, Pfaffenwaldring 57, 70569 Stuttgart, Germany 25. Physikalisches Institut and Center for Integrated Quantum Science and Technology, Universit¨at Stuttgart, Pfaffenwaldring 57, 70569 Stuttgart, Germany 3Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, Campusvej 55, 5230 Odense M, Denmark 4Max Planck Institute for Solid State Research, Heisenbergstraße 1, 70569 Stuttgart, Germany 5Department of Physics and Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA 6Department of Physics of Complex Systems, Weizmann Institute of Science, Rehovot 76100, Israel (Dated: June 7, 2017) The strong interaction between individual Rydberg atoms provides a powerful tool exploited in an ever-growing range of applications in quantum information science, quantum simulation, and ultracold chemistry. One hallmark of the Rydberg interaction is that both its strength and angular dependence can be fine-tuned with great flexibility by choosing appropriate Rydberg states and applying external electric and magnetic fields. More and more experiments are probing this interaction at short atomic distances or with such high precision that perturbative calculations as well as restrictions to the leading dipole-dipole interaction term are no longer sufficient. In this tutorial, we review all relevant aspects of the full calculation of Rydberg interaction potentials. We discuss the derivation of the interaction Hamiltonian from the electrostatic multipole expansion, numerical and analytical methods for calculating the required electric multipole moments, and the inclusion of electromagnetic fields with arbitrary direction. -

RSC Spectroscopy and Dynamics Group Meeting 2020
RSC Spectroscopy and Dynamics Group Meeting 2020 University of Warwick 6th – 8th January 2020 We would like to extend our thanks to the RSC and all of our sponsors for their generous support of this year’s Spectroscopy and Dynamics Group meeting. Please show your support for our sponsors by visiting their trade stands during the coffee breaks and Tuesday’s poster session. Programme All presentation sessions will take place in Meeting Room 2 inside the Radcliffe Conference Centre. Monday 6th January 16:00 - 18:00 Arrival and Registration 18:00 - 19:00 Dinner Dining Room Session 1 Chair: Caroline Dessent 19:00 - 19:05 Welcome Vas Stavros (Warwick) 19:05 - 19:50 Invited tutorial talk Helen Fielding (UCL) 19:50 - 20:35 Invited tutorial talk Tom Penfold (Newcastle) 20:35 - 21:35 SDG AGM Meeting Room 2 Tuesday 7th January 07:00 - 08:30 Breakfast Dining Room* Session 2 Chair: Michael Staniforth 09:00 - 09:45 Invited talk David Osborn (Sandia NL) 09:45 - 10:05 Contributed talk David Kemp (Nottingham) 10:05 - 10:25 Contributed talk Klaudia Gawlas (UCL) 10:25 - 10:45 Contributed talk Preeti Manjari Mishra (RIKEN) 10:45 - 11:15 Tea/Coffee Lounge Session 3 Chair: Jutta Toscano 11:15 - 12:00 Invited talk Sonia Melandri (Bologna) 12:00 - 12:20 Contributed talk Maria Elena Castellani (Durham) 12:20 - 12:40 Contributed talk Javier S. Marti (Imperial) 12:40 - 13:00 Contributed talk Conor Rankine (Newcastle) 13:00 - 14:15 Lunch Dining Room Session 4 Chair: Nat das Neves Rodrigues 14:15 - 14:35 Contributed talk Jacob Berenbeim (York) 14:35 - 14:55 Contributed -

Dynamics of Rydberg Atom Lattices in the Presence of Noise and Dissipation
Dynamics of Rydberg atom lattices in the presence of noise and dissipation D zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Fakultät Mathematik und Naturwissenschaften der Technischen Universität Dresden von Wildan Abdussalam M.Sc. Eingereicht am 19.1.2017 Verteidigt am 07.08.2017 Eingereicht am 19.1.2017 Verteidigt am 07.08.2017 1. Gutachter: Prof. Dr. Jan Michael Rost 2. Gutachter: Prof. Dr. Walter Strunz To my family and teachers Abstract The work presented in this dissertation concerns dynamics of Rydberg atom lattices in the presence of noise and dissipation. Rydberg atoms possess a number of exaggerated properties, such as a strong van der Waals interaction. The interplay of that interaction, coherent driving and decoherence leads to intriguing non-equilibrium phenomena. Here, we study the non-equilibrium physics of driven atom lattices in the pres- ence of decoherence caused by either laser phase noise or strong decay. In the Vrst case, we compare between global and local noise and explore their eUect on the number of ex- citations and the full counting statistics. We Vnd that both types of noise give rise to a characteristic distribution of the Rydberg excitation number. The main method employed is the Langevin equation but for the sake of eXciency in certain regimes, we use a Marko- vian master equation and Monte Carlo rate equations, respectively. In the second case, we consider dissipative systems with more general power- law interactions. We determine the phase diagram in the steady state and analyse its generation dynamics using Monte Carlo rate equations. -

Spectroscopy of Cold Rubidium Rydberg Atoms for Applications in Quantum Information
Spectroscopy of cold rubidium Rydberg atoms for applications in quantum information I.I. Ryabtsev, I.I. Beterov, D.B.Tretyakov, V.M. Entin, E.A. Yakshina Rzhanov Institute of Semiconductor Physics SB RAS, 630090 Novosibirsk, Russia Novosibirsk State University, 630090 Novosibirsk, Russia E-mail: [email protected] Abstract Atoms in highly excited (Rydberg) states have a number of unique properties which make them attractive for applications in quantum information. These are large dipole moments, lifetimes and polarizabilities, as well as strong long-range interactions between Rydberg atoms. Experimental methods of laser cooling and precision spectroscopy enable the trapping and manipulation of single Rydberg atoms and applying them for practical implementation of quantum gates over qubits of a quantum computer based on single neutral atoms in optical traps. In this paper, we give a review of the experimental and theoretical work performed by the authors at the Rzhanov Institute of Semiconductor Physics SB RAS and Novosibirsk State University on laser and microwave spectroscopy of cold Rb Rydberg atoms in a magneto-optical trap and on their possible applications in quantum information. We also give a brief review of studies done by other groups in this area. PACS numbers: 03.67.Lx, 32.70.Jz, 32.80.-t, 32.80.Ee, 32.80.Rm Key words: Rydberg atoms, laser cooling, spectroscopy, quantum information, qubits Journal reference: Physics − Uspekhi 59 (2) 196-208 (2016) DOI: 10.3367/UFNe.0186.201602k.0206 Translated from the original Russian text: Uspekhi Fizicheskikh Nauk 186 (2) 206-219 (2016) DOI: 10.3367/UFNr.0186.201602k.0206 Received 9 November 2015, revised 9 December 2015 2 1. -

Precision Measurements with Rydberg States of Rubidium
Precision Measurements with Rydberg States of Rubidium by Andira Ramos A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Physics) in The University of Michigan 2019 Doctoral Committee: Professor Georg Raithel, Chair Professor Timothy Chupp Professor Duncan Steel Associate Professor Kai Sun Dr. Nicolaas Johannes Van Druten, University of Amsterdam. Andira Ramos [email protected] ORCID iD: 0000-0002-0086-8446 c Andira Ramos 2019 To my wonderful parents. ii ACKNOWLEDGEMENTS Graduate school has not been easy and I am extremely grateful to all the people in my life who have been there to help me through this journey. First, I want to thank my advisor, Georg Raithel, whose endless enthusiasm for physics is contagious. Georg, I truly appreciate the time you spent to answer questions, run calculations and listen to ideas, I know you were always very busy and yet somehow made sure to find time for meetings. You were always patient, understanding and supportive, I am happy to have had you as my research advisor. Getting to work with current and past members of the Raithel group has also been amazing. Thanks to Jamie MacLennan, Ryan Cardman, Michael Viray, Lu Ma and Xiaoxuan Han. Jamie, your support knows no limits and it is a big reason why I have made it this far. Thank you for staying in the lab until the morning hours to keep me company while I ran experiments, thank you for editing applications, emails, and even this thesis (you are hired for life), thank you for the many surprise treats that so often cheered me up when the experiment was not cooperating. -
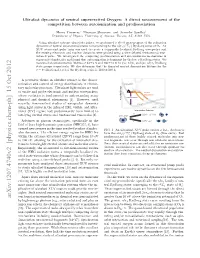
Ultrafast Dynamics of Neutral Superexcited Oxygen: a Direct Measurement of the Competition Between Autoionization and Predissociation
Ultrafast dynamics of neutral superexcited Oxygen: A direct measurement of the competition between autoionization and predissociation Henry Timmers,∗ Niranjan Shivaram, and Arvinder Sandhuy Department of Physics, University of Arizona, Tucson, AZ, 85721 USA. Using ultrafast extreme ultraviolet pulses, we performed a direct measurement of the relaxation 4 − dynamics of neutral superexcited states corresponding to the nlσg(c Σu ) Rydberg series of O2. An XUV attosecond pulse train was used to create a temporally localized Rydberg wavepacket and the ensuing electronic and nuclear dynamics were probed using a time-delayed femtosecond near- infrared pulse. We investigated the competing predissociation and autoionization mechanisms in superexcited molecules and found that autoionization is dominant for the low n Rydberg states. We measured an autoionization lifetime of 92±6 fs and 180±10 fs for (5s; 4d)σg and (6s; 5d)σg Rydberg state groups respectively. We also determine that the disputed neutral dissociation lifetime for the ν = 0 vibrational level of the Rydberg series is 1100 ± 100 fs. A pervasive theme in ultrafast science is the charac- terization and control of energy distributions in elemen- tary molecular processes. Ultrashort light pulses are used Energy to excite and probe electronic and nuclear wavepackets, Neutral whose evolution is fundamental to understanding many Dissociation Autoionization physical and chemical phenomena [1]. However, until Potential recently, time-resolved studies of wavepacket dynamics using light pulses in the infrared (IR), visible, and ultra- violet (UV) regime had predominantly been limited to Internuclear Distance low-lying excited states and femtosecond timescales [2]. Advances in photon technologies, specifically in the ( )+ field of laser high-harmonic generation (HHG)[3, 4], have opened new avenues in the time-resolved studies of molec- FIG. -

Rydberg Molecules and Circular Rydberg States in Cold Atom Clouds
Rydberg molecules and circular Rydberg states in cold atom clouds by David Alexander Anderson A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Applied Physics) in The University of Michigan 2015 Doctoral Committee: Professor Georg A. Raithel, Chair Professor Luming Duan Professor Alex Kuzmich Thomas Pohl, Max Plank Institute for Physics Associate Professor Vanessa Sih ⃝c David A. Anderson 2015 All Rights Reserved For my family ii ACKNOWLEDGEMENTS First I have to thank my advisor Georg Raithel for his continued support, patience, and encouragement during the completion of this dissertation and throughout my time working in the Raithel group. Without his insights, direction, and deep understanding of the subject matter, none of it would have been possible. His enthusiasm for research and insatiable curiosity have been an inspiration in the years I have been fortunate to have spent under his wing. Thank you Georg for giving me the opportunity to learn from you. Over the years I have had the pleasure of working with many former and current members of the Raithel group from whom I have learned a great deal. Many thanks to Sarah Anderson, Yun-Jhih Chen, Lu´ıs Felipe Gon¸calves, Cornelius Hempel, Jamie MacLennan, Stephanie Miller, Kaitlin Moore, Eric Paradis, Erik Power, Andira Ramos, Rachel Sapiro, Andrew Schwarzkopf, Nithiwadee Thaicharoen (Pound), Mallory Traxler, and Kelly Younge, for making my time in the Raithel group such an enjoyable experience. Thank you to my committee members, Georg Raithel, Thomas Pohl, Vanessa Sih, Alex Kuzmich, and Luming Duan, for your support and willingness to serve on my committee. -

Level Shifts of Rubidium Rydberg States Due to Binary Interactions
PHYSICAL REVIEW A 75, 032712 ͑2007͒ Level shifts of rubidium Rydberg states due to binary interactions A. Reinhard, T. Cubel Liebisch, B. Knuffman, and G. Raithel FOCUS Center and Michigan Center for Theoretical Physics, Department of Physics, University of Michigan, Ann Arbor, Michigan 48109, USA ͑Received 16 August 2006; published 14 March 2007; corrected 19 March 2007͒ We use perturbation theory to directly calculate the van der Waals and dipole-dipole energy shifts of pairs of interacting Rb Rydberg atoms for different quantum numbers n, ᐉ, j, and mj, taking into account a large number of perturbing states. Our results can be used to identify good experimental parameters and illuminate important considerations for applications of the “Rydberg-excitation blockade.” We also use the results of the calculation to explain features of previous experimental data on the Rydberg-excitation blockade. To explore control methods for the blockade, we discuss energy shifts due to atom-atom interaction in an external electric field. DOI: 10.1103/PhysRevA.75.032712 PACS number͑s͒: 34.20.Cf, 31.15.Md, 34.60.ϩz, 03.67.Ϫa I. INTRODUCTION external electric field has also been reported ͓15͔. The Ryd- berg blockade has been modeled using many-body quantum Due to their large sizes and polarizabilities, Rydberg at- simulations ͓16,17͔, and the application of the blockade to oms excited from ensembles of laser-cooled ground state at- quantum phase gates ͓18,19͔ has been analyzed. Additional oms interact strongly via resonant dipole-dipole or off- work has been done to characterize Rydberg-atom interac- resonant van der Waals interactions. -

Rydberg States of Atoms and Molecules. Basic Group Theoretical and Topological Analysis
Physics Reports 341 (2001) 173}264 Symmetry, invariants, topology. III Rydberg states of atoms and molecules. Basic group theoretical and topological analysis L. Michel! , B.I. ZhilinskimH " * !Institut des Hautes E! tudes Scientixques, 91440 Bures-sur-Yvette, France "Universite& du Littoral, BP 5526, 59379 Dunkerque Ce& dex, France Contents 1. Introduction 175 3.2. Qualitative description of e!ective 1.1. Dynamical symmetry of Rydberg states 178 Hamiltonians invariant with respect to 2. Groups and their actions appropriate for the continuous subgroups of O(3) 205 Rydberg problem 179 3.3. Qualitative description of e!ective 2.1. Structure of O(4) 179 Hamiltonians invariant with respect to 2.2. The adjoint representation of O(4); induced "nite subgroups of O(3) 210 R" ; action on S S 182 4. Manifestation of qualitative e!ects in physical 2.3. Action of O(3), SO(3), O(3)?T, systems. Hydrogen atom in magnetic and ? ? R SO(3) T, and SO(3) TQ on ; their electric "eld 213 strata, orbits and invariants 185 4.1. Di!erent "eld con"gurations and their 2.4. Invariants of the one-dimensional Lie symmetry 213 subgroups of O(3) acting on R 188 4.2. Quadratic Zeeman e!ect in hydrogen 2.5. One-dimensional Lie subgroups of atom 215 O(3)?T and their invariants 191 4.3. Hydrogen atom in parallel electric and 2.6. Orbits, strata and orbit spaces of the one- magnetic "elds 217 dimensional Lie subgroups of O(3) acting 4.4. Hydrogen atom in orthogonal electric and on R 192 magnetic "elds 223 2.7. -

Interacting Cold Rydberg Atoms: a Toy Many-Body System
Bohr, 1913-2013, S´eminairePoincar´eXVII (2013) 125 { 144 S´eminairePoincar´e Interacting Cold Rydberg Atoms: a Toy Many-Body System Antoine Browaeys and Thierry Lahaye Institut d'Optique Laboratoire Charles Fabry 2 av. A. Fresnel 91127 Palaiseau cedex, France Abstract. This article presents recent experiments where cold atoms excited to Rydberg states interact with each other. It describes the basic properties of Rydberg atoms and their interactions, with the emphasis on the Rydberg blockade mechanism. The paper details a few experimental demonstrations us- ing two individual atoms or atomic ensembles, as well as applications of this \toy many-body system" to quantum information processing using photons. 1 Introduction One of the great challenges in physics is to understand how complexity emerges from the simple underlying physical laws that govern the interactions between con- stituents of matter, like atoms or molecules. Interactions between particles are for the most part well-understood, and the equations of quantum mechanics governing an ensemble of interacting particles are known. However, due to the exponential scaling of the size of the Hilbert space with the number of particles, they are too difficult to be solved beyond a few tens of particles: this is called the many-body problem. One idea to progress is to build in the laboratory some simple toy systems that are ruled by known but unsolved equations and to measure the properties of these systems. In doing so, one can validate or infirm theoretical approaches or models. Furthermore, by developing and harnessing quantum systems, one can envision technological developments. Presently, this quantum engineering has two main applications: quantum information processing and quantum metrology. -

Low-Dielectric-Constant Polyimide Aerogel
University of Birmingham Identification of a new electron-transfer relaxation pathway in photoexcited pyrrole dimers Neville, Simon P.; Kirkby, Oliver M.; Kaltsoyannis, Nikolas; Worth, Graham A.; Fielding, Helen H. DOI: 10.1038/ncomms11357 License: Creative Commons: Attribution (CC BY) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Neville, SP, Kirkby, OM, Kaltsoyannis, N, Worth, GA & Fielding, HH 2016, 'Identification of a new electron- transfer relaxation pathway in photoexcited pyrrole dimers', Nature Communications, vol. 7, 11357. https://doi.org/10.1038/ncomms11357 Link to publication on Research at Birmingham portal Publisher Rights Statement: Neville, S., Kirkby, O., Kaltsoyannis, N. et al. Identification of a new electron-transfer relaxation pathway in photoexcited pyrrole dimers. Nat Commun 7, 11357 (2016). https://doi.org/10.1038/ncomms11357 General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. -

$ C 6 $ Coefficients for Interacting Rydberg Atoms and Alkali-Metal
C6 coefficients for interacting Rydberg atoms and alkali-metal dimers Vanessa Olaya,1 Jes´usP´erez-R´ıos,2 and Felipe Herrera1, 3, ∗ 1Department of Physics, Universidad de Santiago de Chile, Av. Ecuador 3493, Santiago, Chile. 2Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, 14195 Berlin, Germany 3Millennium Institute for Research in Optics MIRO, Chile. (Dated: December 4, 2019) We study the van der Waals interaction between Rydberg alkali-metal atoms with fine structure 2 1 + (n Lj ; L ≤ 2) and heteronuclear alkali-metal dimers in the ground rovibrational state (X Σ ; v = 0, J = 0). We compute the associated C6 dispersion coefficients of atom-molecule pairs involving 133Cs and 85Rb atoms interacting with KRb, LiCs, LiRb, and RbCs molecules. The obtained dispersion coefficients can be accurately fitted to a state-dependent polynomial O(n7) over the range of principal quantum numbers 40 ≤ n ≤ 150. For all atom-molecule pairs considered, 2 2 6 2 Rydberg states n Sj and n Pj result in attractive 1=R potentials. In contrast, n Dj states can give rise to repulsive potentials for specific atom-molecule pairs. The interaction energy at the LeRoy −5 distance approximately scales as n for n > 40. For intermediate values of n . 40, both repulsive and attractive interaction energies in the order of 10 − 100 µK can be achieved with specific atomic and molecular species. The accuracy of the reported C6 coefficients is limited by the quality of the atomic quantum defects, with relative errors ∆C6=C6 estimated to be no greater than 1% on average.