(Turdus Merula) Naturally Infected with Usutu Virus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sindbis Virus Infection in Resident Birds, Migratory Birds, and Humans, Finland Satu Kurkela,*† Osmo Rätti,‡ Eili Huhtamo,* Nathalie Y
Sindbis Virus Infection in Resident Birds, Migratory Birds, and Humans, Finland Satu Kurkela,*† Osmo Rätti,‡ Eili Huhtamo,* Nathalie Y. Uzcátegui,* J. Pekka Nuorti,§ Juha Laakkonen,*¶ Tytti Manni,* Pekka Helle,# Antti Vaheri,*† and Olli Vapalahti*†** Sindbis virus (SINV), a mosquito-borne virus that (the Americas). SINV seropositivity in humans has been causes rash and arthritis, has been causing outbreaks in reported in various areas, and antibodies to SINV have also humans every seventh year in northern Europe. To gain a been found from various bird (3–5) and mammal (6,7) spe- better understanding of SINV epidemiology in Finland, we cies. The virus has been isolated from several mosquito searched for SINV antibodies in 621 resident grouse, whose species, frogs (8), reed warblers (9), bats (10), ticks (11), population declines have coincided with human SINV out- and humans (12–14). breaks, and in 836 migratory birds. We used hemagglutina- tion-inhibition and neutralization tests for the bird samples Despite the wide distribution of SINV, symptomatic and enzyme immunoassays and hemagglutination-inhibition infections in humans have been reported in only a few for the human samples. SINV antibodies were fi rst found in geographically restricted areas, such as northern Europe, 3 birds (red-backed shrike, robin, song thrush) during their and occasionally in South Africa (12), Australia (15–18), spring migration to northern Europe. Of the grouse, 27.4% and China (13). In the early 1980s in Finland, serologic were seropositive in 2003 (1 year after a human outbreak), evidence associated SINV with rash and arthritis, known but only 1.4% were seropositive in 2004. -

Chlamydia Trachomatis Infection Is Driven by Nonprotective Immune Cells That Are Distinct from Protective Populations
Pathology after Chlamydia trachomatis infection is driven by nonprotective immune cells that are distinct from protective populations Rebeccah S. Lijeka,b,1, Jennifer D. Helblea, Andrew J. Olivea,c, Kyra W. Seigerb, and Michael N. Starnbacha,1 aDepartment of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115; bDepartment of Biological Sciences, Mount Holyoke College, South Hadley, MA 01075; and cDepartment of Microbiology and Physiological Systems, University of Massachusetts Medical School, Worcester, MA 01605 Edited by Rafi Ahmed, Emory University, Atlanta, GA, and approved December 27, 2017 (received for review June 23, 2017) Infection with Chlamydia trachomatis drives severe mucosal immu- sequence identity, Chlamydia muridarum, the extent to which the nopathology; however, the immune responses that are required for molecular pathogenesis of C. muridarum represents that of Ct is mediating pathology vs. protection are not well understood. Here, unknown (6). Ct serovar L2 (Ct L2) is capable of infecting the we employed a mouse model to identify immune responses re- mouse upper genital tract when inoculated across the cervix into quired for C. trachomatis-induced upper genital tract pathology the uterus (7, 8) but it does not induce robust immunopathology. and to determine whether these responses are also required for This is consistent with the human disease phenotype caused by Ct L2, bacterial clearance. In mice as in humans, immunopathology was which disseminates to the lymph nodes causing lymphogranuloma characterized by extravasation of leukocytes into the upper genital venereum (LGV) and is not a major cause of mucosal immunopa- thology in the female upper genital tract (uterus and ovaries). tract that occluded luminal spaces in the uterus and ovaries. -

Potential Arbovirus Emergence and Implications for the United Kingdom Ernest Andrew Gould,* Stephen Higgs,† Alan Buckley,* and Tamara Sergeevna Gritsun*
Potential Arbovirus Emergence and Implications for the United Kingdom Ernest Andrew Gould,* Stephen Higgs,† Alan Buckley,* and Tamara Sergeevna Gritsun* Arboviruses have evolved a number of strategies to Chikungunya virus and in the family Bunyaviridae, sand- survive environmental challenges. This review examines fly fever Naples virus (often referred to as Toscana virus), the factors that may determine arbovirus emergence, pro- sandfly fever Sicilian virus, Crimean-Congo hemorrhagic vides examples of arboviruses that have emerged into new fever virus (CCHFV), Inkoo virus, and Tahyna virus, habitats, reviews the arbovirus situation in western Europe which is widespread throughout Europe. Rift Valley fever in detail, discusses potential arthropod vectors, and attempts to predict the risk for arbovirus emergence in the virus (RVFV) and Nairobi sheep disease virus (NSDV) United Kingdom. We conclude that climate change is prob- could be introduced to Europe from Africa through animal ably the most important requirement for the emergence of transportation. Finally, the family Reoviridae contains a arthropodborne diseases such as dengue fever, yellow variety of animal arbovirus pathogens, including blue- fever, Rift Valley fever, Japanese encephalitis, Crimean- tongue virus and African horse sickness virus, both known Congo hemorrhagic fever, bluetongue, and African horse to be circulating in Europe. This review considers whether sickness in the United Kingdom. While other arboviruses, any of these pathogenic arboviruses are likely to emerge such as West Nile virus, Sindbis virus, Tahyna virus, and and cause disease in the United Kingdom in the foresee- Louping ill virus, apparently circulate in the United able future. Kingdom, they do not appear to present an imminent threat to humans or animals. -

Department of Experimental Pathology, Immunology and Microbiology 531
Department of Experimental Pathology, Immunology and Microbiology 531 Department of Experimental Pathology, Immunology and Microbiology Interim Chairperson: Zaatari, Ghazi Vice Chairperson: Matar, Ghassan Professors: Abdelnoor, Alexander; Khouri, Samia; Matar, Ghassan; Sayegh, Mohamed; Zaatari, Ghazi Associate Professor: Rahal, Elias Assistant Professors: Al-Awar, Ghassan; El Hajj, Hiba; Shirinian, Margret; Zaraket, Hassan The Department of Experimental Pathology, Immunology and Microbiology offers courses to medical laboratory sciences (MLSP) students as well as nursing, medical, and graduate students. It offers a graduate program (discipline of Microbiology and Immunology) leading to a master’s degree (MS) or doctoral degree (PhD) in Biomedical sciences. The requirements for admission to the graduate program are stated on page 33 of this catalogue. IDTH 203 The immune System in Health and Disease 37.28; 3 cr. See Interdepartmental Courses. IDTH 205 Microbiology and Infectious Diseases 37.28; 5 cr. See Interdepartmental Courses. MBIM 223 Parasitology for MLSP Students 39.39; 4 cr. Second semester. MBIM 237 Microbiology and Immunology for Nursing Degree Students 32.64; 3 cr. A course on the fundamental aspects of medical microbiology and immunology for nursing students. Second semester. MBIM 260 Elective in Infectious Diseases for Medicine III and IV 0.180 A course on basic evaluation, diagnosis, and management of infectious diseases. One month. MBIM 261 Elective in Immunology for Medicine III and IV 0.180 A course that is an introduction to immunological research and its application to clinical practice. One month. MBIM 310 Basic Immunology 32.32; 3 cr. A course on innate and adaptive immune mechanisms, infection and immunity, vaccination, immune mechanisms in tissue injury and therapeutic immunology. -

Deciphering the Triad of Infection, Immunity and Pathology
INSIGHT DISEASE Deciphering the triad of infection, immunity and pathology The factors which drive and control disease progression can be inferred from mathematical models that integrate measures of immune responses, data from tissue sampling and markers of infection dynamics. FREDERIK GRAW immune actors in the body. Now, in eLife, Related research article Myers MA, Smith Amber Smith and colleagues at St. Jude Child- AP, Lane LC, Moquin DJ, Aogo R, Woolard ren’s Research Hospital, the University of Ten- S, Thomas P, Vogel P, Smith AM. 2021. nessee Health Science Center and the Dynamically linking influenza virus infection Washington University School of Medicine – kinetics, lung injury, inflammation, and dis- including Margaret Myers and Amanda Smith as ease severity. eLife 10:e68864. doi: 10. joing first authors – report how viral infection, 7554/eLife.68864 counteracting immune responses and lung pathology interact as mice fight off influenza A (Myers et al., 2021). First, the team tracked how viral load and the number of CD8+ T cells, an important immune fever, a cough, a splitting headache... actor that helps to clear infected cells, pro- Being sick often comes with tell-tale gressed over time. In combination with mathe- A signs which worsen as the disease pro- matical models, these measurements allowed gresses and tissues become damaged. These Myers et al. to estimate several parameters that symptoms result from complex interactions reflect the pace at which the virus replicates, the between the infecting pathogen, the inflamma- strength of the immune response, and the inter- tion process, and the response from the immune actions between these processes. -

Overview of Pathology and Its Related Disciplines - Soheir Mahmoud Mahfouz
MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz OVERVIEW OF PATHOLOGY AND ITS RELATED DISCIPLINES Soheir Mahmoud Mahfouz Cairo University, Kasr El Ainy Hospital, Egypt Keywords: Pathology, Pathology disciplines, Pathology techniques, Ancillary diagnostic methods, General Pathology, Special Pathology Contents 1. Introduction 1.1 Pathology coverage 1.1.1 Etiology and Pathogenesis of a Disease 1.1.2 Manifestations of Disease (Lesions) 1.1.3 Phases Of A Disease Process (Course) 1.2 Physician’s approach to patient 1.3 Types of pathologists and affiliated specialties 1.4 Role of pathologist 2. Pathology and its related disciplines 2.1 Cytology 2.1.1 Cytology Samples 2.1.2 Technical Aspects 2.1.3 Examination of Sample and Diagnosis 3. Pathology techniques and ancillary diagnostic methods 3.1 Macroscopic pathology 3.2 Light Microscopy 3.3 Polarizing light microscopy 3.4 Electron microscopy (EM) 3.5 Confocal Microscopy 3.6 Frozen section 3.7 Cyto/histochemistry 3.8 Immunocyto/histochemical methods 3.9 Molecular and genetic methods of diagnosis 3.10 Quantitative methods 4. Types of tests used in pathology 4.1 DiagnosticUNESCO tests – EOLSS 4.2 Quantitative tests 4.3 Prognostic tests 5. The scope of SAMPLEpathology & its main divisions CHAPTERS 6. Conclusions Glossary Bibliography Biographical sketch Summary Pathology is the science of disease. It deals with deviations from normal body function and ©Encyclopedia of Life Support Systems (EOLSS) MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz structure. Many disciplines are involved in the study of disease, as it is necessary to understand the complex causes and effects of various disorders that affect the organs and body as a whole. -

Competition Between Usutu Virus and West Nile Virus During Simultaneous and Sequential Infection of Culex Pipiens Mosquitoes
Emerging Microbes & Infections ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/temi20 Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes Haidong Wang , Sandra R. Abbo , Tessa M. Visser , Marcel Westenberg , Corinne Geertsema , Jelke J. Fros , Constantianus J. M. Koenraadt & Gorben P. Pijlman To cite this article: Haidong Wang , Sandra R. Abbo , Tessa M. Visser , Marcel Westenberg , Corinne Geertsema , Jelke J. Fros , Constantianus J. M. Koenraadt & Gorben P. Pijlman (2020) Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culexpipiens mosquitoes, Emerging Microbes & Infections, 9:1, 2642-2652, DOI: 10.1080/22221751.2020.1854623 To link to this article: https://doi.org/10.1080/22221751.2020.1854623 © 2020 The Author(s). Published by Informa View supplementary material UK Limited, trading as Taylor & Francis Group, on behalf of Shanghai Shangyixun Cultural Communication Co., Ltd Published online: 14 Dec 2020. Submit your article to this journal Article views: 356 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=temi20 Emerging Microbes & Infections 2020, VOL. 9 https://doi.org/10.1080/22221751.2020.1854623 ORIGINAL ARTICLE Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes Haidong Wanga, Sandra R. Abboa, -

Usutu Virus: an Emerging Flavivirus in Europe
Viruses 2015, 7, 219-238; doi:10.3390/v7010219 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Usutu Virus: An Emerging Flavivirus in Europe 1,2,3 1,2,3 1,2,3 1,2,3 1,2,3 Usama Ashraf , Jing Ye , Xindi Ruan , Shengfeng Wan , Bibo Zhu and Shengbo Cao 1,2,3,* 1 State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, Hubei 430070, China; E-Mails: [email protected] (U.A.); [email protected] (J.Y.); [email protected] (X.R.); [email protected] (S.W.); [email protected] (B.Z.) 2 Laboratory of Animal Virology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, Hubei 430070, China 3 Laboratory of development of Veterinary Diagnostic Products, Ministry of Agriculture, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, Hubei 430070, China * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-278-728-2608. Academic Editor: Karyn Johnson Received: 11 November 2014 / Accepted: 13 January 2015 / Published: 19 January 2015 Abstract: Usutu virus (USUV) is an African mosquito-borne flavivirus belonging to the Japanese encephalitis virus serocomplex. USUV is closely related to Murray Valley encephalitis virus, Japanese encephalitis virus, and West Nile virus. USUV was discovered in South Africa in 1959. In Europe, the first true demonstration of circulation of USUV was reported in Austria in 2001 with a significant die-off of Eurasian blackbirds. In the subsequent years, USUV expanded to neighboring countries, including Italy, Germany, Spain, Hungary, Switzerland, Poland, England, Czech Republic, Greece, and Belgium, where it caused unusual mortality in birds. -
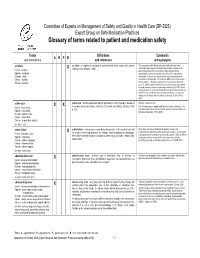
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -
Pathology: a Career in Medicine the Study of the Nature of Disease, Its Causes, Processes, Development, and Consequences
PATHOLOGY A Career in Medicine The Intersociety Council for Pathology Information (ICPI) www.pathologytraining.org 2015 Pathology: A Career in Medicine The study of the nature of disease, its causes, processes, development, and consequences. Pathology is the medical specialty that provides a scientific foundation for medical practice The pathologist is a physician who specializes in the diagnosis and management of human disease by laboratory methods. Pathologists function in three broad areas: as diagnosticians, as teachers, and as investigators. Fundamental to the discipline of pathology is the need to integrate clinical information with physiological, biochemical and molecular laboratory studies, together with observations of tissue alterations. Pathologists in hospital and clinical laboratories practice as consultant physicians, developing and applying knowledge of tissue and laboratory analyses to assist in the diagnosis and treatment of individual patients. As teachers, they impart this knowledge of disease to their medical colleagues, to medical students, and to trainees at all levels. As scientists, they use the tools of laboratory science in clinical studies, disease models, and other experimental systems, to advance the understanding and treatment of disease. Pathology has a special appeal to those who enjoy solving disease-related problems, using technologies based upon fundamental sciences ranging from biophysics to molecular genetics, as well as tools from the more traditional disciplines of anatomy, biochemistry, pharmacology, physiology and microbiology. The Pathologist in Patient Care The pathologist uses diagnostic and screening tests to identify and interpret the changes that characterize different diseases in the cells, tissues, and fluids of the body. Anatomic pathology involves the analysis of the A biosample robot prepares specimens for gross and microscopic structural changes caused by testing disease in tissues and cells removed during biopsy procedures, in surgery, or at autopsy. -

Assessment of Vector Competence of UK Mosquitoes for Usutu Virus of African Origin Luis M
Hernández-Triana et al. Parasites & Vectors (2018) 11:381 https://doi.org/10.1186/s13071-018-2959-5 SHORTREPORT Open Access Assessment of vector competence of UK mosquitoes for Usutu virus of African origin Luis M. Hernández-Triana1*, Maria Fernández de Marco1, Karen L. Mansfield1, Leigh Thorne1, Sarah Lumley1,2,3, Denise Marston1, Anthony A. Fooks1,4 and Nick Johnson1,2 Abstract Background: Usutu virus (USUV) is an emerging zoonotic virus originally from sub-Saharan Africa. It has been introduced into Europe on multiple occasions, causing substantial mortality within the Eurasian blackbird (Turdus merula) population. It is transmitted by the mosquito species Culex pipiens in Europe and Africa. Vector competence studies indicate that European strains of USUV are readily transmitted by indigenous Cx. pipiens. However, there is limited information on the ability of an African strain to infect European mosquitoes. Methods: We evaluated the ability of African strain SAAR-1776 to infect two lines of Cx. pipiens colonised within the United Kingdom (UK). Mosquitoes were fed blood meals containing this virus and maintained at 25 °C for up to 21 days. Individual mosquitoes were tested for the presence of virus in the body, legs and an expectorate saliva sample. Changes to the consensus of the virus genome were monitored in samples derived from infected mosquitoes using amplicon based next generation sequencing. Results: Infection, dissemination and the presence of virus in saliva in one mosquito line was observed, but no evidence for dissemination in the second mosquito line. This suggests a strong barrier to infection in UK Cx. pipiens for this strain of USUV. -

Cytopathology (Path Cyto)
path cyto 1 Pathology: Cytopathology Page updated: August 2020 This section contains information to assist providers in billing for pathology procedures related to cytopathology services. Pap Smear Tests Taking a Papanicolaou (Pap) smear sample is considered part of a pelvic examination and is not separately reimbursable. The Pap smear test is reimbursable only to the provider who performs and reads the Pap smear and issues the written report. These tests include up to three smears for Pap tests for cancer screening and/or a qualitative report on the patient’s level of estrogen. Modifiers TC and 26 Providers may use modifier TC to bill cervical or vaginal Pap smear results. When a smear is billed with modifier 26, it is reimbursable only to a hospital pathologist whose service is not covered by the hospital. Submitting a claim on one line without a modifier indicates that both the professional and technical components of the service were provided. Clinical or Hospital Laboratory Pap smears examined in a clinical or hospital laboratory can be reimbursed without a modifier only if both the professional and technical components are performed in the laboratory. Billing Restrictions CPT® code 88120 will not be reimbursed if billed in conjunction with codes 88121 or 88365; CPT code 88121 will not be reimbursed if billed in conjunction with codes 88120 or 88365. CPT codes 88142, 88143, 88147, 88148, 88150, 88152, 88153, 88164, 88165, 88166 or 88167, 88174 or 88175 may be used to bill cervical or vaginal Pap smear tests and to report physician interpretation services. Part 2 – Cytopathology path cyto 2 Page updated: August 2020 Recommended frequencies are as follows: ‹‹Recommended Frequencies CPT Codes›› CPT Codes Recommended Frequency 88142, 88143, 88147, 88148, 88150, One in 30 days 88152, 88153, 88174, 88175 88164 thru 88167 One in one year Frequency limits apply to claims billed by any provider, for the same recipient.