Marrubium Vulgare L., Herba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malacological Diversity on Four Lamiaceae in the Region of Tlemcen
Journal of Plant Sciences and Crop Protection Volume 1 | Issue 1 Review Article Open Access Malacological diversity on four Lamiaceae in the region of Tlemcen (Northwest of Algeria) Damerdji A* Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, Algeria *Corresponding author: Damerdji A, Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, 13000, Algeria, E-mail: [email protected] Citation: Damerdji A (2018) Malacological diversity on four Lamiaceae in the region of Tlemcen (North- west of Algeria). J Plant Sci Crop Protec 1(1): 106. doi: 10.15744/2639-3336.1.106 Received Date: March 07, 2018 Accepted Date: July 25, 2018 Published Date: July 27, 2018 Abstract The region of Tlemcen is located in the north-west Algeria. Tends arid climate leads to a degradation of vegetation in open formation, where are found the doum the diss and broom.... Other aromatic species are considered: rosemary, thyme, lavender and horehound. By their morphological and botanical four aromatic species belonging to the Labiatae family. We propose an approach to achieve diversity malacofauna identified on these Lamiaceae. These latters are certainly a nutritional source for this malacological fauna. For this, an inventory is made in different stations. Malacological wealth of thyme is estimated at 19, that the rosemary to 18, on 14 and lavender to last, that the horehound 7. It includes four families namely Milacidae the Sphincterochilidae the Helicidae and Subulinidae. Milacidae are present only in horehound and lavender stations. On the other hand, the Sphincterochilidae, namely Sphincterochila candidissima, is absent on horehound and lavander. -

The Insect Fauna Associated with Horehound (Marrubium Vulgare L
Plant Protection Quarterly Vol.15(1) 2000 21 belonging to eight orders were found feeding on the plant (Figure 2, Table 2). The insect fauna associated with horehound The insects included 12 polyphagous spe- (Marrubium vulgare L.) in western Mediterranean cies (44%), 8 oligophagous species (30%) and 7 monophagous species (26%). At the Europe and Morocco: potential for biological control larval stage, there were five root-feeding in Australia species (22%), one stem-boring species (4%), nine leaf-feeding species (39%), eight flower, ovary or seed feeding species A Jean-Louis Sagliocco , Keith Turnbull Research Institute, Victorian (34%). Based on adult feeding behaviour Department of Natural Resources and Environment, CRC for Weed there was one root-boring species (74%), Management Systems, PO Box 48, Frankston, Victoria 3199, Australia. six leaf-feeding species (40%) and eight A Previous address: CSIRO European Laboratory, Campus International de species feeding on flowers or ovaries or Baillarguet, 34980 Montferrier sur Lez, Cedex, France. seeds (53%). Wheeleria spilodactylus (Curtis) Summary were preserved. Immature stages were (Lepidoptera: Pterophoridae) Marrubium vulgare L. (Lamiaceae) was kept with fresh plant material until the Wheeleria spilodactylus was abundant at surveyed in western Mediterranean Eu- adult stage for identification. Insects most sites in France and Spain, and had rope and Morocco to identify the phy- were observed either in the field or the been recorded feeding on M. vulgare tophagous insect fauna associated with laboratory to confirm that they fed on the (Gielis 1996) and Ballota nigra (Bigot and this weed and to select species having plant. Insects were sent to museum spe- Picard 1983). -

Marrubium Vulgare L
A WEED REPORT from the book Weed Control in Natural Areas in the Western United States This WEED REPORT does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This WEED REPORT is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Marrubium vulgare L. White horehound Family: Lamiaceae Range: Nearly all states, including all western states, except perhaps North Dakota. Found almost worldwide. Habitat: Pastures, fields, roadsides, rangeland, disturbed natural areas, waste places, ditches, and other disturbed places. Most often in dry places. White horehound is especially common in overgrazed areas. Origin: Native to Eurasia. White horehound was once cultivated as a medicinal herb and for flavoring candy. Impacts: Expands range during drought conditions, especially under heavy grazing. Once established, it can outcompete native vegetation and form dense stands in annual grasslands. Livestock avoid consuming the bitter-tasting foliage, and the plant thrives in the absence of competition with other vegetation. California Invasive Plant Council (Cal-IPC) Inventory: Limited Invasiveness White horehound is a cool-season perennial to 2 ft tall, often looking like a low shrub. It has densely hairy white-woolly stems, thick and square in cross-section, which mostly branch near the base of the plant. Its leaves are aromatic, opposite on the stems, ovate to nearly round, 0.5 to 2.5 inches long, with round-toothed margins. -
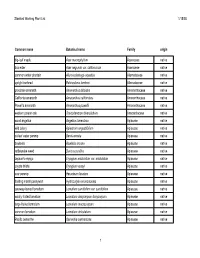
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -

NVEO 2017, Volume 4, Issue 4, Pages 14-27
Nat. Volatiles & Essent. Oils, 2017: 4(4): 14-27 Celep & Dirmenci REVIEW Systematic and Biogeographic overview of Lamiaceae in Turkey Ferhat Celep1,* and Tuncay Dirmenci2 1 Mehmet Akif Ersoy mah. 269. cad. Urankent Prestij Konutları, C16 Blok, No: 53, Demetevler, Ankara, TURKEY 2 Biology Education, Necatibey Education Faculty, Balıkesir University, Balıkesir, TURKEY *Corresponding author. E-mail: [email protected] Abstract Lamiaceae is the third largest family based on the taxon number and fourth largest family based on the species number in Turkey. The family has 48 genera and 782 taxa (603 species, 179 subspecies and varieties), 346 taxa (271 species, 75 subspecies and varieties) of which are endemic (ca. 44%) (data updated 1th February 2017) in the country. There are also 23 hybrid species, 19 of which are endemic (82%). The results proven that Turkey is one of the centers of diversity for Lamiaceae in the Old World. In addition, Turkey has about 10% of all Lamiaceae members in the World. The largest five genera in the country based on the taxon number are Stachys (118 taxa), Salvia (107 taxa), Sideritis (54 taxa), Phlomis (53 taxa) and Teucrium (49 taxa). According to taxon number, five genera with the highest endemism ratio are Dorystaechas (1 taxon, 100%), Lophantus (1 taxon, 100%), Sideritis (54 taxa, 74%), Drymosiphon (9 taxa, 67%), and Marrubium (27 taxa, 63%). There are two monotypic genera in Turkey as Dorystaechas and Pentapleura. Turkey sits on the junction of three phytogeographic regions with highly diverse climate and the other ecologic features. Phytogeographic distribution of Turkish Lamiaceae taxa are 293 taxa in the Mediterranean (37.4%), 267 taxa in the Irano-Turanian (36.7%), 90 taxa in the Euro-Siberian (Circumboreal) phytogeographic region, and 112 taxa in Unknown or Multiregional (14.3%) phytogeographical elements. -

Plant Section Introduction
Re-introduction Practitioners Directory - 1998 RE-INTRODUCTION PRACTITIONERS DIRECTORY 1998 Compiled and Edited by Pritpal S. Soorae and Philip J. Seddon Re-introduction Practitioners Directory - 1998 © National Commission for Wildlife Conservation and Development, 1998 Printing and Publication details Legal Deposit no. 2218/9 ISBN: 9960-614-08-5 Re-introduction Practitioners Directory - 1998 Copies of this directory are available from: The Secretary General National Commission for Wildlife Conservation and Development Post Box 61681, Riyadh 11575 Kingdom of Saudi Arabia Phone: +966-1-441-8700 Fax: +966-1-441-0797 Bibliographic Citation: Soorae, P. S. and Seddon, P. J. (Eds). 1998. Re-introduction Practitioners Directory. Published jointly by the IUCN Species Survival Commission’s Re-introduction Specialist Group, Nairobi, Kenya, and the National Commission for Wildlife Conservation and Development, Riyadh, Saudi Arabia. 97pp. Cover Photo: Arabian Oryx Oryx leucoryx (NWRC Photo Library) Re-introduction Practitioners Directory - 1998 CONTENTS FOREWORD Professor Abdulaziz Abuzinadai PREFACE INTRODUCTION Dr Mark Stanley Price USING THE DIRECTORY ACKNOWLEDGEMENTS PART A. ANIMALS I MOLLUSCS 1. GASTROPODS 1.1 Cittarium pica Top Shell 1.2 Placostylus ambagiosus Flax Snail 1.3 Placostylus ambagiosus Land Snail 1.4 Partula suturalis 1.5 Partula taeniata 1.6 Partula tahieana 1.7 Partula tohiveana 2. BIVALVES 2.1 Freshwater Mussels 2.2 Tridacna gigas Giant Clam II ARTHROPODS 3. ORTHOPTERA 3.1 Deinacrida sp. Weta 3.2 Deinacrida rugosa/parva Cook’s Strait Giant Weta Re-introduction Practitioners Directory - 1998 3.3 Gryllus campestris Field Cricket 4. LEPIDOPTERA 4.1 Carterocephalus palaemon Chequered Skipper 4.2 Lycaena dispar batavus Large Copper 4.3 Lycaena helle 4.4 Lycaeides melissa 4.5 Papilio aristodemus ponoceanus Schaus Swallowtail 5. -

Horehound in the Garden
Revised May 2020 HG/Garden/2008-01pr Horehound in the Garden Becky Barton and Dan Drost Vegetable Specialist Summary Horehound (Marrubium vulgare) is a tender drought hardy perennial and a member of the mint family. Horehound prefers full sun and well drained soils. Plant in early spring, either from seed or transplants. Seeds are slow to germinate; therefore, sow shallow and keep moist for optimum emergence. Thin to 10 inches apart once established and harvest when the plant starts to bloom. Once established, horehound is very drought tolerant. Like all members of the mint family, it spreads rapidly and can become weedy. Horehound is used to make teas, candies and cough drops. Varieties Silver horehound has a whiter flower and has woollier leaves than the common horehound. Spanish horehound has a pink flower. Most plants are hardy to USDA Zone 4. Consult local specialty nurseries or seed catalogs for additional varieties. Horehound will survive winters in most areas of Soil preparation: Before planting, till the Utah, but may need protection in colder areas of top 6 to 8 inches of soil, but do not enrich the soil the state. with fertilizer or compost. How to Grow Plants: Horehound can be started from seeds or cuttings in the early spring. Seeds should Soils: Horehound grows in most soils types be sown just below the surface (¼ inch deep) about especially poor, dry and neglected soils. Horehound 3 weeks before the frost free date for your area. does best in full sun and sandy well-drained soil. Seeds are very slow to germinate. -

Indiana Medical History Museum Guide to the Medicinal Plant Garden
Indiana Medical History Museum Guide to the Medicinal Plant Garden Garden created and maintained by Purdue Master Gardeners of Marion County IMHM Medicinal Plant Garden Plant List – Common Names Trees and Shrubs: Arborvitae, Thuja occidentalis Culver’s root, Veronicastrum virginicum Black haw, Viburnum prunifolium Day lily, Hemerocallis species Catalpa, Catalpa bignonioides Dill, Anethum graveolens Chaste tree, Vitex agnus-castus Elderberry, Sambucus nigra Dogwood, Cornus florida Elecampane, Inula helenium Elderberry, Sambucus nigra European meadowsweet, Queen of the meadow, Ginkgo, Ginkgo biloba Filipendula ulmaria Hawthorn, Crateagus oxycantha Evening primrose, Oenothera biennis Juniper, Juniperus communis False Solomon’s seal, Smilacina racemosa Redbud, Cercis canadensis Fennel, Foeniculum vulgare Sassafras, Sassafras albidum Feverfew, Tanacetum parthenium Spicebush, Lindera benzoin Flax, Linum usitatissimum Witch hazel, Hamamelis virginiana Foxglove, Digitalis species Garlic, Allium sativum Climbing Vines: Golden ragwort, Senecio aureus Grape, Vitis vinifera Goldenrod, Solidago species Hops, Humulus lupulus Horehound, Marrubium vulgare Passion flower, Maypop, Passiflora incarnata Hyssop, Hyssopus officinalis Wild yam, Dioscorea villosa Joe Pye weed, Eupatorium purpureum Ladybells, Adenophora species Herbaceous Plants: Lady’s mantle, Alchemilla vulgaris Alfalfa, Medicago sativa Lavender, Lavendula angustifolia Aloe vera, Aloe barbadensis Lemon balm, Melissa officinalis American skullcap, Scutellaria laterifolia Licorice, Glycyrrhiza -

Complete Iowa Plant Species List
!PLANTCO FLORISTIC QUALITY ASSESSMENT TECHNIQUE: IOWA DATABASE This list has been modified from it's origional version which can be found on the following website: http://www.public.iastate.edu/~herbarium/Cofcons.xls IA CofC SCIENTIFIC NAME COMMON NAME PHYSIOGNOMY W Wet 9 Abies balsamea Balsam fir TREE FACW * ABUTILON THEOPHRASTI Buttonweed A-FORB 4 FACU- 4 Acalypha gracilens Slender three-seeded mercury A-FORB 5 UPL 3 Acalypha ostryifolia Three-seeded mercury A-FORB 5 UPL 6 Acalypha rhomboidea Three-seeded mercury A-FORB 3 FACU 0 Acalypha virginica Three-seeded mercury A-FORB 3 FACU * ACER GINNALA Amur maple TREE 5 UPL 0 Acer negundo Box elder TREE -2 FACW- 5 Acer nigrum Black maple TREE 5 UPL * Acer rubrum Red maple TREE 0 FAC 1 Acer saccharinum Silver maple TREE -3 FACW 5 Acer saccharum Sugar maple TREE 3 FACU 10 Acer spicatum Mountain maple TREE FACU* 0 Achillea millefolium lanulosa Western yarrow P-FORB 3 FACU 10 Aconitum noveboracense Northern wild monkshood P-FORB 8 Acorus calamus Sweetflag P-FORB -5 OBL 7 Actaea pachypoda White baneberry P-FORB 5 UPL 7 Actaea rubra Red baneberry P-FORB 5 UPL 7 Adiantum pedatum Northern maidenhair fern FERN 1 FAC- * ADLUMIA FUNGOSA Allegheny vine B-FORB 5 UPL 10 Adoxa moschatellina Moschatel P-FORB 0 FAC * AEGILOPS CYLINDRICA Goat grass A-GRASS 5 UPL 4 Aesculus glabra Ohio buckeye TREE -1 FAC+ * AESCULUS HIPPOCASTANUM Horse chestnut TREE 5 UPL 10 Agalinis aspera Rough false foxglove A-FORB 5 UPL 10 Agalinis gattingeri Round-stemmed false foxglove A-FORB 5 UPL 8 Agalinis paupercula False foxglove -

Plant List for VC54, North Lincolnshire
Plant List for Vice-county 54, North Lincolnshire 3 Vc61 SE TA 2 Vc63 1 SE TA SK NORTH LINCOLNSHIRE TF 9 8 Vc54 Vc56 7 6 5 Vc53 4 3 SK TF 6 7 8 9 1 2 3 4 5 6 Paul Kirby, 31/01/2017 Plant list for Vice-county 54, North Lincolnshire CONTENTS Introduction Page 1 - 50 Main Table 51 - 64 Summary Tables Red Listed taxa recorded between 2000 & 2017 51 Table 2 Threatened: Critically Endangered & Endangered 52 Table 3 Threatened: Vulnerable 53 Table 4 Near Threatened Nationally Rare & Scarce taxa recorded between 2000 & 2017 54 Table 5 Rare 55 - 56 Table 6 Scarce Vc54 Rare & Scarce taxa recorded between 2000 & 2017 57 - 59 Table 7 Rare 60 - 61 Table 8 Scarce Natives & Archaeophytes extinct & thought to be extinct in Vc54 62 - 64 Table 9 Extinct Plant list for Vice-county 54, North Lincolnshire The main table details all the Vascular Plant & Stonewort taxa with records on the MapMate botanical database for Vc54 at the end of January 2017. The table comprises: Column 1 Taxon and Authority 2 Common Name 3 Total number of records for the taxon on the database at 31/01/2017 4 Year of first record 5 Year of latest record 6 Number of hectads with records before 1/01/2000 7 Number of hectads with records between 1/01/2000 & 31/01/2017 8 Number of tetrads with records between 1/01/2000 & 31/01/2017 9 Comment & Conservation status of the taxon in Vc54 10 Conservation status of the taxon in the UK A hectad is a 10km. -

Plant Species Richness and Composition of a Habitat Island
Biodiversity Data Journal 8: e48704 doi: 10.3897/BDJ.8.e48704 Research Article Plant species richness and composition of a habitat island within Lake Kastoria and comparison with those of a true island within the protected Pamvotis lake (NW Greece) Alexandros Papanikolaou‡‡, Maria Panitsa ‡ Division of Plant Biology, Department of Biology, University of Patras, Patras, Greece Corresponding author: Maria Panitsa ([email protected]) Academic editor: Gianniantonio Domina Received: 22 Nov 2019 | Accepted: 07 Jan 2020 | Published: 15 Jan 2020 Citation: Papanikolaou A, Panitsa M (2020) Plant species richness and composition of a habitat island within Lake Kastoria and comparison with those of a true island within the protected Pamvotis lake (NW Greece). Biodiversity Data Journal 8: e48704. https://doi.org/10.3897/BDJ.8.e48704 Abstract Lake Kastoria is one of the potentially “ancient” Balkan lakes that has a great environmental importance and ecological value, attracts high touristic interest and is under various anthropogenic pressures. It belongs to a Natura 2000 Special Protection Area and a Site of Community Interest. The city of Kastoria is located at the western part of the lake and just next to it, towards the centre of the lake, is a peninsula, a habitat island. In the framework of research concerning the flora of lake islands of Greece, one of the main objectives of the present study is to fill a gap concerning plant species richness of the habitat island within the protected Lake Kastoria, which is surrounded by the lake except for its north-western part where the border of the city of Kastoria is located. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA.