Pharmaceutical Innovation in the APAC Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report and Financial Statements
Annual Report and Financial Statements for the year ended 31 December 2018 Dimensional Funds ICVC Authorised by the Financial Conduct Authority No marketing notification has been submitted in Germany for the following Funds of Dimensional Funds ICVC: Global Short-Dated Bond Fund International Core Equity Fund International Value Fund United Kingdom Core Equity Fund United Kingdom Small Companies Fund United Kingdom Value Fund Accordingly, these Funds must not be publicly marketed in Germany. Table of Contents Dimensional Funds ICVC General Information* 2 Investment Objectives and Policies* 3 Authorised Corporate Directors’ Investment Report* 6 Incorporation and Share Capital* 10 The Funds 10 Fund Cross-Holdings 10 Authorised Status* 10 Regulatory Disclosure* 10 Potential Implications of Brexit* 10 Responsibilities of the Authorised Corporate Director 11 Responsibilities of the Depositary 11 Report of the Depositary to the Shareholders 11 Directors' Statement 11 Independent Auditors’ Report to the Shareholders of Dimensional Funds ICVC 12 The Annual Report and Financial Statements for each of the below sub-funds (the “Funds”); Emerging Markets Core Equity Fund Global Short-Dated Bond Fund International Core Equity Fund International Value Fund United Kingdom Core Equity Fund United Kingdom Small Companies Fund United Kingdom Value Fund are set out in the following order: Fund Information 14 Portfolio Statement* 31 Statement of Total Return 149 Statement of Change in Net Assets Attributable to Shareholders 149 Balance Sheet 150 Notes to the Financial Statements 151 Distribution Tables 168 Remuneration Disclosures (unaudited)* 177 Supplemental Information (unaudited) 178 * These collectively comprise the Authorised Corporate Directors’ (“ACD”) Report. Dimensional Fund Advisors Ltd. Annual Report and Financial Statements, 31 December 2018 1 Dimensional Funds ICVC General Information Authorised Corporate Director (the “ACD”): Dimensional Fund Advisors Ltd. -

FTSE Korea 30/18 Capped
2 FTSE Russell Publications 19 August 2021 FTSE Korea 30/18 Capped Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) Alteogen 0.19 KOREA Hyundai Engineering & Construction 0.35 KOREA NH Investment & Securities 0.14 KOREA AmoreG 0.15 KOREA Hyundai Glovis 0.32 KOREA NHN 0.07 KOREA Amorepacific Corp 0.65 KOREA Hyundai Heavy Industries 0.29 KOREA Nong Shim 0.08 KOREA Amorepacific Pfd. 0.08 KOREA Hyundai Marine & Fire Insurance 0.13 KOREA OCI 0.17 KOREA BGF Retail 0.09 KOREA Hyundai Merchant Marine 1.02 KOREA Orion 0.21 KOREA BNK Financial Group 0.18 KOREA Hyundai Mipo Dockyard 0.15 KOREA Ottogi 0.06 KOREA Celltrion Healthcare 0.68 KOREA Hyundai Mobis 1.53 KOREA Paradise 0.07 KOREA Celltrion Inc 2.29 KOREA Hyundai Motor 2.74 KOREA Posco 1.85 KOREA Celltrion Pharm 0.24 KOREA Hyundai Motor 2nd Pfd. 0.33 KOREA Posco Chemical 0.32 KOREA Cheil Worldwide 0.14 KOREA Hyundai Motor Pfd. 0.21 KOREA Posco International 0.09 KOREA CJ Cheiljedang 0.3 KOREA Hyundai Steel 0.33 KOREA S1 Corporation 0.13 KOREA CJ CheilJedang Pfd. 0.02 KOREA Hyundai Wia 0.13 KOREA Samsung Biologics 0.92 KOREA CJ Corp 0.11 KOREA Industrial Bank of Korea 0.22 KOREA Samsung C&T 0.94 KOREA CJ ENM 0.15 KOREA Kakao 3.65 KOREA Samsung Card 0.08 KOREA CJ Logistics 0.12 KOREA Kangwon Land 0.23 KOREA Samsung Electro-Mechanics 0.81 KOREA Coway 0.36 KOREA KB Financial Group 1.78 KOREA Samsung Electronics 25.36 KOREA Daewoo Engineering & Construction 0.12 KOREA KCC Corp 0.12 KOREA Samsung Electronics Pfd. -

Scientific Committee on Vaccine Preventable Diseases and Scientific Committee on Emerging and Zoonotic Diseases
Scientific Committee on Emerging and Zoonotic Disease and Scientific Committee on Vaccine Preventable Diseases Consensus Interim Recommendations on the Use of COVID-19 Vaccines in Hong Kong (As of Jan 7, 2021) Introduction The ongoing COVID-19 pandemic causes a significant disease burden worldwide. In Hong Kong, cases and outbreaks continue to be reported. To reduce the impacts of COVID-19 on health and society, vaccines against COVID-19 is considered an important public health tool for containing the pandemic in the medium and long term. On 7 January 2021, the Scientific Committee on Emerging and Zoonotic Diseases (SCEZD), the Scientific Committee on Vaccine Preventable Diseases (SCVPD), and the Expert Advisory Panel to Chief Executive (EAP) reviewed the latest scientific evidence on the epidemiology and clinical features of COVID-19, published data on the COVID- 19 vaccines be procured by the Hong Kong SAR Government, local data as well as overseas recommendations/practices, and provides recommendations on the population groups and circumstances for the use of COVID-19 vaccines in Hong Kong. COVID-19 Vaccines 2. At the meeting held on 13 August 2020, the joint SCEZD and SCVPD together with the EAP reviewed the then scientific development of COVID 19 vaccines and prioritization of target groups for COVID 19 vaccines in Hong Kong. The meeting recommended that vaccine procurement would be aimed at the whole Hong Kong population in the long run. In anticipation of a limited supply at the early stage when vaccines are available, a phased approach has to be taken with certain priority groups of the local population identified to be vaccinated first, in order to reduce morbidity and mortality and maintain essential services. -

Confectionery Fresh and Fermented Dairy Pharmaceuticals Processed
Creating new value as “Food and Health” professionals (TSE Section 1: 2269) To enrich the daily lives of customers of all ages by providing them with tastiness and enjoyment as well as products that contribute to their physical and emotional well-being Fresh and Fermented Dairy Confectionery Pharmaceuticals Antibacterial Generic drugs drugs Yogurt Drinking Chocolate/ Gummy milk Chocolate snacks Drugs for CNS disorders Processed Food Nutrition Ice cream Cheese Infant Enteral Sports nutrition formula formula Consolidated Performance and Share Price in Recent Years Net sales Operating income (%) ROE Payout ratio / DOE Share price (July 2014 – October 2017) (JPY1 bn) (JPY1 bn) 20 (%) (%) (JPY) 90 1,200 90 6 16 10,000 7,500 800 60 12 60 4 DOE 8 5,000 400 30 30 2 2,500 4 Payout ratio 0 0 0 0 0 0 3 3 3 3 3 3 3 3 3 3 3 3 E E E 7 0 1 4 7 0 1 4 7 0 1 4 7 0 / / / / 3 3 3 3 / / / / / / / / E / / / / / / / / / / / / / / 3 3 3 1 1 1 1 4 5 6 7 4 5 6 7 4 5 6 7 / / 3 / 4 5 5 5 6 6 6 7 7 7 / / / / 4 5 6 7 / 1 1 1 1 1 1 1 1 1 1 1 1 8 8 8 ' ' ' ' 1 4 1 1 1 5 1 1 1 6 1 1 1 7 ' ' ' ' ' ' ' ' 1 1 1 1 ' ' ' ' ' ' ' ' ' ' 8 ' ' ' ' 1 1 1 1 1 1 1 ' ' ' ' ' ' ' 1 ' History Major Shareholders Number of Percentage of (as of September 30, 2017) shares held total shares Meiji Sugar Co., Ltd., the origin of the Meiji Group, was established. -

Immunogenicity and Safety of a Third Dose, and Immune Persistence Of
medRxiv preprint doi: https://doi.org/10.1101/2021.07.23.21261026; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Immunogenicity and safety of a third dose, and immune persistence of 2 CoronaVac vaccine in healthy adults aged 18-59 years: interim results 3 from a double-blind, randomized, placebo-controlled phase 2 clinical 4 trial 5 6 Hongxing Pan MSc1*, Qianhui Wu MPH2*, Gang Zeng Ph.D.3*, Juan Yang Ph.D.1, Deyu 7 Jiang MSc4, Xiaowei Deng MSc2, Kai Chu MSc1, Wen Zheng BSc2, Fengcai Zhu M.D.5†, 8 Hongjie Yu M.D. Ph.D.2,6,7†, Weidong Yin MBA8† 9 10 Affiliations 11 1. Vaccine Evaluation Institute, Jiangsu Provincial Center for Disease Control and 12 Prevention, Nanjing, China 13 2. School of Public Health, Fudan University, Key Laboratory of Public Health Safety, 14 Ministry of Education, Shanghai, China 15 3. Clinical Research Department, Sinovac Biotech Co., Ltd., Beijing, China 16 4. Covid-19 Vaccine Department, Sinovac Life Sciences Co., Ltd., Beijing, China 17 5. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China 18 6. Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, 19 Shanghai, China 20 7. Department of Infectious Diseases, Huashan Hospital, Fudan University, 21 Shanghai, China 22 8. Sinovac Biotech Co., Ltd., Beijing, China NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Pharma China0409-285.P65
TM C O N T E N T S ○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○ ○○○ I s s u e Editorial Mentholatum sues XiAn Meichen over Market Dynamics Brew New Order for trademark infringements 14 Chinese Pharma 2 API/Bulk Drugs 32 News in Focus Huaxing expands capacity of Amoxicillin MOH releases results of the 4th and 6-APA 14 APRIL National Health Service Survey 4 NCPG/DSM likely to build major The Market antibiotic API facility in Changchun 14 2 0 0 9 Data Snaptshot: Chinese OTC Shandong Antibiotics granted Germany healthcare market 5 GMP certification 15 Nicholas Hall reports slower growth Hisun sees profits up sharply in 2008 15 of OTC GI market in China 5 Changzhou Yabang-QH builds new In This Issue Industry News cGMP API plant with help from J&J 15 Chinese pharma industry perfor- Shijiazhuang Pharma receives EDQM MNCs report another year of mance in 2008 and outlook in 2009 5 certification for Vitamin B12 15 sharp revenue growth in China MNCs report another year of sharp India likely to investigate China’s 6-APA 15 Many MNCs experienced growth 20% revenue growth in China 6 MOC issues list of authorized exporters or more in China last year amid global MNCs positions for the huge diabetes of ephedrine drugs 15 slowdown P6 market potential in China 6 Data Snapshot - COS certifications and Harbin Pharma reconsiders Chinese producers likely to emerge DMFs held by Chinese companies 16 overseas IPO as global players of recombinant Chinese API in 2008 – Output and Export In preparation, Harbin Pharmaceutical human insulin 6 Volumes Down 16 Group is working on major and minor M&A is likely to intensify for retail Product and R&D News acquisitions this year P8 pharmacy sector in 2009 7 AOB to initiate clinical trials of TCM drug Pfizer opens new sterile facility Guangzhou introduces online for UI in the U.S. -
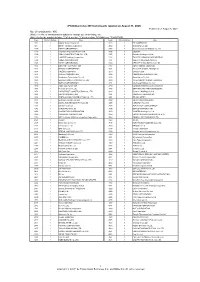
JPX-Nikkei Index 400 Constituents (Applied on August 31, 2021) Published on August 6, 2021 No
JPX-Nikkei Index 400 Constituents (applied on August 31, 2021) Published on August 6, 2021 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3048 1 BIC CAMERA INC. 1417 1 MIRAIT Holdings Corporation 3064 1 MonotaRO Co.,Ltd. 1605 1 INPEX CORPORATION 3088 1 Matsumotokiyoshi Holdings Co.,Ltd. 1719 1 HAZAMA ANDO CORPORATION 3092 1 ZOZO,Inc. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3107 1 Daiwabo Holdings Co.,Ltd. 1721 1 COMSYS Holdings Corporation 3116 1 TOYOTA BOSHOKU CORPORATION 1766 1 TOKEN CORPORATION 3141 1 WELCIA HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1802 1 OBAYASHI CORPORATION 3167 1 TOKAI Holdings Corporation 1803 1 SHIMIZU CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1808 1 HASEKO Corporation 3244 1 Samty Co.,Ltd. 1812 1 KAJIMA CORPORATION 3254 1 PRESSANCE CORPORATION 1820 1 Nishimatsu Construction Co.,Ltd. 3288 1 Open House Co.,Ltd. 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3289 1 Tokyu Fudosan Holdings Corporation 1824 1 MAEDA CORPORATION 3291 1 Iida Group Holdings Co.,Ltd. 1860 1 TODA CORPORATION 3349 1 COSMOS Pharmaceutical Corporation 1861 1 Kumagai Gumi Co.,Ltd. 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3382 1 Seven & I Holdings Co.,Ltd. 1881 1 NIPPO CORPORATION 3391 1 TSURUHA HOLDINGS INC. 1893 1 PENTA-OCEAN CONSTRUCTION CO.,LTD. -

2018 Unaudited Semiannual Report
BOCOM INTERNATIONAL CHINA DYNAMIC FUND (A sub-fund of BOCOM International Fund) SEMI-ANNUAL REPORT (UNAUDITED) FOR THE SIX MONTHS ENDED 30 JUNE 2018 SEMI-ANNUAL REPORT (UNAUDITED) FOR THE SIX MONTHS ENDED 30 JUNE 2018 BOCOM INTERNATIONAL CHINA DYNAMIC FUND (A sub-fund of BOCOM International Fund) Contents Pages Management and Administration 1 Report of the Manager to the Unitholders 2 Statement of Financial Position (Unaudited) 3 Investment Portfolio (Unaudited) 4 – 5 Statement of Movements in Investment Portfolio (Unaudited) 6 – 9 SEMI-ANNUAL REPORT (UNAUDITED) FOR THE SIX MONTHS ENDED 30 JUNE 2018 BOCOM INTERNATIONAL CHINA DYNAMIC FUND (A sub-fund of BOCOM International Fund) MANAGEMENT AND ADMINISTRATION Manager Directors of the Manager BOCOM International Asset Management Limited TAN Yueheng 9/F, Man Yee Building LI Ying 68 Des Voeux Road Central CHENG Chuange Central Hong Kong Trustee and Registrar Bank of Communications Trustee Limited 1/F, Far East Consortium Building 121 Des Voeux Road Central Central Hong Kong PRC Custodian HSBC Bank (China) Company Limited 33/F, HSBC Building, Shanghai ifc 8 Century Avenue, Pudong Shanghai Legal Counsel to the Manager Deacons 5/F, Alexandra House 18 Chater Road Central Hong Kong Auditor PricewaterhouseCoopers 21/F, Edinburgh Tower 15 Queen’s Road Central Hong Kong - 1 - SEMI-ANNUAL REPORT (UNAUDITED) FOR THE SIX MONTHS ENDED 30 JUNE 2018 BOCOM INTERNATIONAL CHINA DYNAMIC FUND (A sub-fund of BOCOM International Fund) REPORT OF THE MANAGER TO THE UNITHOLDERS The A-share market under-performance in 1H2018 can be attributed to two main factors: 1) the United States entered an interest rate hike cycle, which caused wide concern about the emerging market’s debt problems. -

COVID-19: China Medical Supply Chains and Broader Trade Issues
COVID-19: China Medical Supply Chains and Broader Trade Issues Updated December 23, 2020 Congressional Research Service https://crsreports.congress.gov R46304 SUMMARY R46304 COVID-19: China Medical Supply Chains and December 23, 2020 Broader Trade Issues Karen M. Sutter, The outbreak of Coronavirus Disease 2019 (COVID-19), first in China, and then Coordinator globally, including in the United States, has drawn attention to the ways in which the Specialist in Asian Trade U.S. economy depends on manufacturing and supply chains based in China. This report and Finance aims to assess current developments and identify immediate and longer range China trade issues for Congress. Andres B. Schwarzenberg Analyst in International An area of particular concern to Congress has been U.S. shortages in medical supplies— Trade and Finance including personal protective equipment (PPE) and pharmaceuticals—as the United States stepped up efforts to contain the COVID-19 pandemic with limited domestic Michael D. Sutherland stockpiles and insufficient U.S. industrial capacity. Because of China’s role as a global Analyst in International supplier of PPE, medical devices, antibiotics, and active pharmaceutical ingredients, Trade and Finance reduced exports from China led to shortages of critical medical supplies in the United States. Exacerbating the situation, in early February 2020, the Chinese government nationalized control of the production and distribution of medical supplies in China— directing all production for domestic use—and directed the bureaucracy and Chinese industry to secure supplies from the global market. Once past the initial peak of its COVID-19 outbreak, the Chinese government appears to have prioritized certain countries and selectively released some medical supplies for overseas delivery. -

Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

China Pharmaceutical Newsletter
Volume VII 2011 CHINA PHARMACEUTICAL ڵNEWSLETTER З֡Ԛ哦֡ଢ଼рࡗЗ แྼჯ)ཀৄDžᄱᆶ၌ࠅິ SFDA Commissioner Shao Mingli SFDA Deputy Commissioner Wu Zhen of NPC Standing Committee attended the met with new Cuban Ambassador to meets the Head of Iran's Innovation and meeting. Chen Zhu, the Health Minister China On September 29, 2011, Shao Technology Cooperation Center On the & Chairman of the Forum attended the Mingli, Commissioner of SFDA met morning of September 6, 2011, Wu Zhen, forum and delivered a speech. with Mr. ALberto Jesus Blanco Silva, the SFDA Deputy Commissioner, met with the Chen Zhu said in his speech, through new Cuban Ambassador Extraordinary visiting Mr. Hamidreza Amirinia, Head of 30 years of reform and opening up, and Plenipotentiary to China, and his Innovation and Technology Cooperation China's GDP has maintained a 10% entourage in Beijing. The two sides Center of Iran. Both parties exchanged growth in 30 consecutive years, and held in-depth discussions on further views on enhancing mutual exchanges and created an economic miracle. In 2010, strengthening the bilateral cooperation in understanding, and promoting cooperation China's GDP had ranked second in the WKH¿HOGVRIELRORJLFDl products and drug LQWKH¿HOGRIWUDGLWLRQDO&KLQHVHPHGLFLQH world. In the 21st century, the Chinese safety supervision. (September 30, 2011) and biopharmaceuticals. (September 8, 2011) Government pays more attention to social SFDA Deputy Commissioner Wu development, taking the alleviation of SFDA Deputy Commissioner Bian Zhen meets the delegation of MHLW On poverty and improvement of health care, Zhenjia attends the APEC LSIF Drug the morning of August 23, 2011, Wu Zhen, education, housing, and employment, etc. -

VANGUARD INTERNATIONAL EQUITY INDEX FUNDS Form N-Q
SECURITIES AND EXCHANGE COMMISSION FORM N-Q Quarterly schedule of portfolio holdings of registered management investment company filed on Form N-Q Filing Date: 2018-09-28 | Period of Report: 2018-07-31 SEC Accession No. 0000932471-18-007167 (HTML Version on secdatabase.com) FILER VANGUARD INTERNATIONAL EQUITY INDEX FUNDS Mailing Address Business Address PO BOX 2600 PO BOX 2600 CIK:857489| IRS No.: 000000000 | State of Incorp.:DE | Fiscal Year End: 1031 V26 V26 Type: N-Q | Act: 40 | File No.: 811-05972 | Film No.: 181093806 VALLEY FORGE PA 19482 VALLEY FORGE PA 19482 6106691000 Copyright © 2018 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM N-Q QUARTERLY SCHEDULE OF PORTFOLIO HOLDINGS OF REGISTERED MANAGEMENT COMPANY Investment Company Act file number: 811-05972 Name of Registrant: VANGUARD INTERNATIONAL EQUITY FUNDS Address of Registrant: P.O. Box 2600 Valley Forge, PA 19482 Name and address of agent for service: Anne E. Robinson, Esquire P.O. Box 876 Valley Forge, PA 19482 Date of fiscal year end: October 31 Date of reporting period: July 31, 2018 Item 1: Schedule of Investments Vanguard Pacific Stock Index Fund Schedule of Investments (unaudited) As of July 31, 2018 Market Value Shares ($000) Common Stocks (99.6%)1 Australia (16.6%) Commonwealth Bank of Australia 1,856,264 103,370 BHP Billiton Ltd. 3,386,626 88,447 Westpac Banking Corp. 3,610,167 79,036 CSL Ltd. 475,901 69,628 Australia & New Zealand Banking Group Ltd.