4.4.1.6 Vascular Access: Venous Cutdown, Great Saphenous Vein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Massive Transfusion and Control of Hemorrhage in the Trauma Patient
Massive Transfusion and Control of Hemorrhage in the Trauma Patient N A L T R A I O U T M A A N R C E A T R N E I I Based on Special ITACCS Seminar Panels. The International Trauma Anesthesia and Critical Care Society (ITACCS) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) for physicians. This CME activity was planned and produced in accordance with the ACCME Essentials. ITACCS designates this CME activity for 15 credit hours in Category 1 of the Physicians Recognition Award of the American Medical Association. I CME QUESTIONS INCLUDED JANUARY 2003 LEARNING OBJECTIVES OF THE MONOGRAPH Chapter 6 Atraumatic blood salvage and autotransfusion in trauma and surgery .................................. Page 17 Sherwin V. Kevy, MD, and Robert Brustowicz, MD, Trans- After completion of this activity, the participant will be able to: fusion Service, Children’s Hospital Department of Anes- thesia, Harvard Medical School, Boston, Massachusetts 1. Evaluate the etiology and pathophysiology of traumatic shock. 2. Describe the management of massive transfusion in the trauma patient. Section III: Transfusion: Clinical Practice 3. Discuss the clinical indications and problems related to the use of blood, blood components, hemostatic agents, oxygen-carrying vol- Chapter 7 Current practices in blood and blood ume expanders, and venous thromboembolism prophylaxis. component therapy ....................................... Page 18 Charles E. Smith, MD, FRCPC, Department of Anesthesi- EDITORS ology, MetroHealth Medical Center, Case Western Reserve University School of Medicine, Cleveland, Ohio Charles E. Smith, MD, FRCPC, Professor of Anesthesiology, Chapter 8 Immunomodulatory effects of transfusion .. Page 22 MetroHealth Medical Center, Case Western Reserve University School David T. -

General Surgery Residency Curriculum
General Surgery Educational Goals & Objectives General surgeons are specialists in the evaluation and treatment of patients with injuries, deformities, or medical illnesses that require operative intervention. They may diagnose and treat acute and chronic disorders of the breast, endocrine system, gastrointestinal tract, and skin and soft tissue and may go on to further training in a particular area, including such specialties as bariatrics, cardiothoracic surgery, colorectal surgery, oncology, pediatrics, surgical critical care, transplant, trauma, and vascular surgery. Our General Surgery Residency at Community Memorial Hospital will provide the resident with a strong foundation in basic science, including anatomy, biology, immunology, pathology, and physiology, with special expertise in the clinical care of patients in the community. The goal of the residency is to provide exposure to a broad base of surgical interventions and to encourage critical thinking, such that graduates can provide compassionate care at the forefront of knowledge in General Surgery. Residents spend the majority of their initial time in General Surgery, with additional rotations in Anesthesia and Critical Care to develop foundational skills upon which to base further training. Subsequent rotations in General Surgery and its subspecialties hone technical skills and promote dedicated study of General Surgery in both the inpatient and outpatient settings. Focus will be on learning normal and abnormal anatomy, understanding the natural history of surgical disease (untreated, treated medically, and treated surgically), and gaining expertise in multiple procedural skills. Comprehensive experience in pre-and post-operative care as well as exceptional operative training ensures that our residents achieve excellence in the diagnosis and management of surgical disease. -

High Ligation of Sapheno-Femoral Junction and Thermal Ablation For
High ligation of sapheno-femoral junction and thermal ablation for lower limb Ann Ital Chir, 2020 91, 1: 61-64 primary varicosity in day hospital setting pii: S0003469X20030262 Epub Ahead of Print 2 June 2019 free reading: www.annitalchir.com Luciano Izzo*, Federico Pugliese*, Gorizio Pieretti°, Sara Izzo*, Paolo Izzo* With collaboration of : Gaetano Florio***, Mauro Del Papa***, Daniela Messineo** *Department of Surgery “Pietro Valdoni”, Policlinico “Umberto I”, “Sapienza” University, Rome, Italy **Department of Radiological Sciences, Oncology and Pathology, “Sapienza” University, Rome, Italy ***L.P. Delfino Hospital, Department of General Surgery, Colleferro, Rome, Italy °Multidisciplinary Department of Medical-Surgical and Dental Specialties, Plastic Surgery Unit, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy High ligation of sapheno-femoral junction and thermal ablation for lower limb primary varicosity in day hospital setting AIM: The traditional surgical treatment for lower limb primary varicosity has been for a long time high ligation of sapheno-femoral junction and stripping of great saphenous vein. Surgery, however, has been frustrated by postoperative pains and discomfort and recurrences so that it has been challenged by minimally invasive endovenous techniques such as laser treatment and radiofrequency ablation. The aim of the article is to assess the feasibility, in a day hospital set- ting, of a combined approach to greater saphenous vein reflux: high ligation of sapheno-femoral junction and thermal treatment of the great sapenous vein. METHODS: A retrospective analysis on 95 patients treated with high ligation and thermal ablation at our institution from January 2009 to July 2017 was performed, assessing duration of surgery, post-operative pain and analgesics require- ments, early complications and resumption of activities. -

Clinical Review: Vascular Access for Fluid Infusion in Children
Clinical review: Vascular access for fluid infusion in children • Nikolaus A Haas Critical Care20048:478 https://doi.org/10.1186/cc2880 © BioMed Central Ltd 2004 • Published: 3 June 2004 Abstract The current literature on venous access in infants and children for acute intravascular access in the routine situation and in emergency or intensive care settings is reviewed. The various techniques for facilitating venous cannulation, such as application of local warmth, transillumination techniques and epidermal nitroglycerine, are described. Preferred sites for central venous access in infants and children are the external and internal jugular veins, the subclavian and axillary veins, and the femoral vein. The femoral venous cannulation appears to be the most safe and reliable technique in children of all ages, with a high success and low complication rates. Evidence from the reviewed literature strongly supports the use of real- time ultrasound techniques for venous cannulation in infants and children. Additionally, in emergency situations the intraosseous access has almost completly replaced saphenous cutdown procedures in children and has decreased the need for immediate central venous access. Keywords • central venous access • child • epidermal nitroglycerine • intraosseous • transillumination • venous cutdown Introduction Nothing can be more difficult, time consuming and frustrating than obtaining vascular access in the paediatric patient. This was best described by Orlowski in 1984 [1], who stated, 'My kingdom for an intravenous line'. -

I Have Reviewed the DOP/Roster Provided to Me by MSS and Confirm As Indicated Below
ATRIUM HEALTH REAPPOINTMENT DELINEATION OF PRIVILEGES SPECIALTY OF EMERGENCY MEDICINE I have reviewed the DOP/Roster provided to me by MSS and confirm as indicated below: My DOP is accurate and reflects privileges relevant to my current practice I have listed privileges that should be removed: Printed Name: Signature: Date: If your roster indicates that you hold any of the privileges listed below, you must provide the maintenance criteria as described, in order to maintain the privilege. Your maintenance criteria and attestation must be returned together. Maintenance Criteria for Continued Special Privileges (CEMD-1(a-c): • Emergency Ultrasound – Biliary (Cholecystitis and Cholelithiasis) • Emergency Ultrasound – Urinary Tract (Hydronephrosis and bladder size) • Emergency Ultrasound – DVT The Physician must submit a minimum of ten (10) cases over the past two (2) years for each ultrasound privileges held, based on acceptable results of ongoing professional practice evaluation and outcomes, and five (5) ultrasound related Category I or II CME hours over the past two (2) years to reapply for special privileges. This will be reviewed at the time of reappointment. Physicians who would like to continue to hold any special privileges but are unable to document the minimal number will be requested to voluntarily withdraw their request for such privileges and to complete the necessary proctoring forms. Maintenance Criteria for Continued Special Privileges (CEMD-1(d-e): • Emergency Ultrasound – Thoracic • Emergency Ultrasound - Bowel The Physician must submit a minimum of two (2) cases over the past two (2) years for each ultrasound privileges held, based on acceptable results of ongoing professional practice evaluation and outcomes to reapply for special privileges. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Venous Cutdown Versus the Seldinger Technique for Placement of Totally Implantable Venous Access Ports (Protocol)
Cochrane Database of Systematic Reviews Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports (Protocol) Hsu CCT, Kwan GNC, van Driel ML, Rophael JA Hsu CCT, Kwan GNC, van Driel ML, Rophael JA. Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD008942. DOI: 10.1002/14651858.CD008942. www.cochranelibrary.com Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 3 METHODS ...................................... 3 REFERENCES ..................................... 5 CONTRIBUTIONSOFAUTHORS . 6 DECLARATIONSOFINTEREST . 6 SOURCESOFSUPPORT . 6 Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports (Protocol) i Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Protocol] Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports Charlie C-T Hsu1, Gigi NC Kwan2, Mieke L van Driel3, John A Rophael4 1The Alfred Hospital, Prahran, Australia. 2Box Hill Hospital, Box Hill, Australia. 3Faculty of Health -

Pub 100-04 Medicare Claims Processing
Department of Health & CMS Manual System Human Services (DHHS) Pub 100-04 Medicare Claims Centers for Medicare & Processing Medicaid Services (CMS) Transmittal 784 Date: DECEMBER 16, 2005 Change Request 4238 SUBJECT: January 2006 Outpatient Prospective Payment System Code Editor (OPPS OCE) Specifications Version 7.0 I. SUMMARY OF CHANGES: This instruction is to inform the Fiscal Intermediaries that the January 2006 Outpatient Prospective Payment System Outpatient Code Editor (OPPS OCE) specifications have been updated with new additions, deletions, and changes. NEW/REVISED MATERIAL EFFECTIVE DATE: January 1, 2006 IMPLEMENTATION DATE: January 3, 2006 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R = REVISED, N = NEW, D = DELETED – Only One Per Row. R/N/D Chapter / Section / SubSection / Title III. FUNDING: No additional funding will be provided by CMS; Contractor activities are to be carried out within their FY 2006 operating budgets. IV. ATTACHMENTS: Recurring Update Notification *Unless otherwise specified, the effective date is the date of service. Attachment – Recurring Update Notification Pub. 100-04 Transmittal: 784 Date: December 16, 2005 Change Request 4238 SUBJECT: January 2006 Outpatient Prospective Payment System Code Editor (OPPS OCE) Specifications Version 7.0 I. GENERAL INFORMATION A. Background: This notification reflects specifications that were issued for the October revision of the OPPS OCE (Version 6.3). -
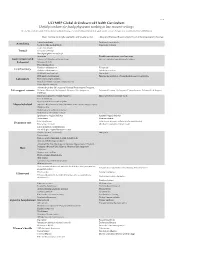
UCFMRP Global & Undeserved Health Curriculum: Useful Procedures for Family Physicians Working in Low-Resource Settings
Oct-14 UCFMRP Global & Undeserved Health Curriculum: Useful procedures for family physicians working in low-resource settings This list was created to guide FM resident procedural training, it is not mandatory but can help guide elective options. For questions contact [email protected] Basic Training: Generally applicable and broadly useful. Advanced Training: Requires higher level of training and/or technology Topical anesthesia Peripheral nerve block Anesthesia Local anesthesia/field block Conscious sedation Tooth Extractions Dental Temporary Fillings Dental peripheral nerve block Anoscopy Flexible sigmoidoscopy or colonoscopy Gastrointestinal & Excision of thrombosed hemorrhoid Incision and drainage of perirectal abcess Colorectal Nasogastric tube Fecal disimpaction Newborn circumcision Vasectomy Genitourinary Bladder catheterization Adult circumcision KOH (skin and vaginal) Gram Stain ECG electrode placement Fine needle aspiration of superficial masses & lymphatics Laboratory Urine microscopic analysis Peripheral smear evaluation (malaria, etc) Urine dipstick analysis Advanced Cardiac Life Support, Neonatal Resuscitation Program, Life support courses Pediatric Advanced Life Support, Advanced Life Support in Advanced Trauma Life Support, Comprehensive Advanced Life Support Obstetrics Initial management of simple fractures Upper and lower extremity casts Closed reduction Upper and lower extremity splints Musculoskeletal Injection & aspiration of knee, shoulder, troch. bursa, trigger fingers, trigger points Reduction of shoulder -

Ultrasound Guidance Versus Anatomical Landmarks for Internal Jugular Vein Catheterization (Review)
Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization (Review) Brass P,Hellmich M, Kolodziej L, Schick G, Smith AF This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 1 http://www.thecochranelibrary.com Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 8 OBJECTIVES ..................................... 9 METHODS ...................................... 10 RESULTS....................................... 14 Figure1. ..................................... 15 Figure2. ..................................... 18 Figure3. ..................................... 19 Figure4. ..................................... 22 Figure5. ..................................... 23 ADDITIONALSUMMARYOFFINDINGS . 27 DISCUSSION ..................................... 30 AUTHORS’CONCLUSIONS . 33 ACKNOWLEDGEMENTS . 34 REFERENCES ..................................... 34 CHARACTERISTICSOFSTUDIES . 41 DATAANDANALYSES. 112 Analysis 1.1. Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 1 Complication rate -

Sepsis Prevention: Vascular Access Care and Laboratory Testing in the Intensive Care Unit
SEPSIS PREVENTION: VASCULAR ACCESS CARE AND LABORATORY TESTING IN THE INTENSIVE CARE UNIT JASSIN M. JOURIA, MD DR. JASSIN M. JOURIA IS A MEDICAL DOCTOR, PROFESSOR OF ACADEMIC MEDICINE, AND MEDICAL AUTHOR. HE GRADUATED FROM ROSS UNIVERSITY SCHOOL OF MEDICINE AND HAS COMPLETED HIS CLINICAL CLERKSHIP TRAINING IN VARIOUS TEACHING HOSPITALS THROUGHOUT NEW YORK, INCLUDING KING’S COUNTY HOSPITAL CENTER AND BROOKDALE MEDICAL CENTER, AMONG OTHERS. DR. JOURIA HAS PASSED ALL USMLE MEDICAL BOARD EXAMS, AND HAS SERVED AS A TEST PREP TUTOR AND INSTRUCTOR FOR KAPLAN. HE HAS DEVELOPED SEVERAL MEDICAL COURSES AND CURRICULA FOR A VARIETY OF EDUCATIONAL INSTITUTIONS. DR. JOURIA HAS ALSO SERVED ON MULTIPLE LEVELS IN THE ACADEMIC FIELD INCLUDING FACULTY MEMBER AND DEPARTMENT CHAIR. DR. JOURIA CONTINUES TO SERVES AS A SUBJECT MATTER EXPERT FOR SEVERAL CONTINUING EDUCATION ORGANIZATIONS COVERING MULTIPLE BASIC MEDICAL SCIENCES. HE HAS ALSO DEVELOPED SEVERAL CONTINUING MEDICAL EDUCATION COURSES COVERING VARIOUS TOPICS IN CLINICAL MEDICINE. RECENTLY, DR. JOURIA HAS BEEN CONTRACTED BY THE UNIVERSITY OF MIAMI/JACKSON MEMORIAL HOSPITAL’S DEPARTMENT OF SURGERY TO DEVELOP AN E-MODULE TRAINING SERIES FOR TRAUMA PATIENT MANAGEMENT. DR. JOURIA IS CURRENTLY AUTHORING AN ACADEMIC TEXTBOOK ON HUMAN ANATOMY & PHYSIOLOGY. Abstract When patients are brought to the Intensive Care Unit, extensive laboratory testing and monitoring is often considered necessary in order to diagnose and treat critical conditions. However, laboratory tests and monitoring are not without risk. Results can be misleading, and the testing itself can be harmful, such as potentially causing anemia and infection. Health professionals need to take a sensible approach to laboratory testing and monitoring for patients in the Intensive Care Unit, focusing on the benefits and risks of each procedure and being mindful of the probability of disease. -

Trouble Shooting in Endovenous Treatment
Trouble Shooting in Endovenous Treatment Dr. Wisoot Wongklahan Thai Phlebology Society https://www.facebook.com/เส้นเลอื ดขอดรกั ษาไดไ้ ม่ ต้องผา่ ตดั Line ID : drwis By. Vein Clinic 31 JAN 2020 Thai Phlebology Society 1 Trouble Shooting in Endovenous Treatment General health condition 1. Carefully taken medical history 2. Procedure is elective so patients should be healthy (ASA I,II) 3. Patients on long-term oral anticoagulation safely treat with ETA 4. Previous operation 5. Obesity 2 Trouble Shooting in Endovenous Treatment Contraindications Absolute contraindications • Acute deep vein thrombosis (DVT), • Acute superficial phlebitis, • Acute infections at puncture sites (infection should be treated first), • Deep venous obstruction if the vein to be treated is a functional collateral. Relative contraindications • Immobile or hardly ambulating patients • Concomitant significant peripheral arterial disease with (ankle:brachial pressure index < 0.5 or an absolute ankle pressure < 60 mmHg) • Patients unable to undergo local anesthesia • Elevated thromboembolic risk including documented thrombophilia and history of previous DVT. • Pregnancy • Patients with significant uncompensated leg edema • Uncontrolled severe diseases. 3 Trouble Shooting in Endovenous Treatment 4 Treatment Plan Discussed with the patient about findings • Treatment option : ½ legs, stage treatment • Patient expectation : Recurrent, Bulging, Ulcer, Leg swelling, Hyperpigmentation, Pain • Cost • Risk, Side effect • Informed consent • Instruction for Pre-op & Post-op care/time