Policies and Procedure Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Changes in Serum Micrornas After Anti-IL-5 Biological Treatment of Severe Asthma
International Journal of Molecular Sciences Article Changes in Serum MicroRNAs after Anti-IL-5 Biological Treatment of Severe Asthma Manuel J. Rial 1,2, José A. Cañas 2,3 , José M. Rodrigo-Muñoz 2,3 , Marcela Valverde-Monge 1 , Beatriz Sastre 2,3 , Joaquín Sastre 1,3 and Victoria del Pozo 2,3,* 1 Allergy Unit, Hospital Universitario Fundación Jiménez Díaz, 28040 Madrid, Spain; [email protected] (M.J.R.); [email protected] (M.V.-M.); [email protected] (J.S.) 2 Department of Immunology, IIS-Fundación Jiménez Díaz, 28040 Madrid, Spain; [email protected] (J.A.C.); [email protected] (J.M.R.-M.); [email protected] (B.S.) 3 CIBER de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-9155-048-91 Abstract: There is currently enough evidence to think that miRNAs play a role in several key points in asthma, including diagnosis, severity of the disease, and response to treatment. Cells release different types of lipid double-membrane vesicles into the extracellular microenvironment, including exosomes, which function as very important elements in intercellular communication. They are capable of distributing genetic material, mRNA, mitochondrial DNA, and microRNAs (miRNAs). Serum miRNA screening was performed in order to analyze possible changes in serum miRNAs in 10 patients treated with reslizumab and 6 patients with mepolizumab after 8 weeks of treatment. The expression of miR-338-3p was altered after treatment (p < 0.05), although no significant differences between reslizumab and mepolizumab were found. Bioinformatic analysis showed that miR-338-3p regulates important pathways in asthma, such as the MAPK and TGF-β signaling pathways and the Citation: Rial, M.J.; Cañas, J.A.; biosynthesis/degradation of glucans (p < 0.05). -

MEP-AST Mepolizumab Versus Placebo for Asthma
MEP-AST Mepolizumab versus placebo for asthma Mepolizumab versus placebo for asthma Review information Review type: Intervention Review number: MEP-AST Authors Colin Powell1, Stephen J Milan2, Kerry Dwan3, Lynne Bax4, Nicola Walters5 1Department of Child Health, Institute of Molecular and Experimental Medicine, Cardiff University School of Medicine, Cardiff, UK 2Lancaster Health Hub, Lancaster University, Lancaster, UK 3Department of Biostatistics, University of Liverpool, Liverpool, UK 4Lancashire Care NHS Foundation Trust, Preston, UK 5Chest Unit, St George's University Hospitals NHS Foundation Trust, London, UK Citation example: Powell C, Milan SJ, Dwan K, Bax L, Walters N. Mepolizumab versus placebo for asthma. Cochrane Database of Systematic Reviews 2015 , Issue 7 . Art. No.: CD010834. DOI: 10.1002/14651858.CD010834.pub2 . Contact person Colin Powell Senior Lecturer Department of Child Health Institute of Molecular and Experimental Medicine, Cardiff University School of Medicine Cardiff UK E-mail: [email protected] Dates Assessed as Up-to-date:19 November 2014 Date of Search: 19 November 2014 Next Stage Expected: 6 June 2017 Protocol First Published:Issue 11 , 2013 Review First Published: Issue 7 , 2015 Last Citation Issue: Issue 7 , 2015 What's new Date Event Description History Date Event Description Abstract Background Mepolizumab is a human monoclonal antibody against interleukin-5 (IL-5), the main cytokine involved in the activation of eosinophils, which in turn causes airway inflammation. Recent studies have suggested these agents may have a role in reducing exacerbations and improving health-related quality of life (HRQoL). There are no recommendations for the use of mepolizumab in adults or children in the recent update of the BTS/SIGN guidelines (BTS/SIGN 2014). -

Asthma Agents
APPROVED PA Criteria Initial Approval Date: July 10, 2019 Revised Date: January 20, 2021 CRITERIA FOR PRIOR AUTHORIZATION Asthma Agents BILLING CODE TYPE For drug coverage and provider type information, see the KMAP Reference Codes webpage. MANUAL GUIDELINES Prior authorization will be required for all current and future dose forms available. All medication-specific criteria, including drug-specific indication, age, and dose for each agent is defined in Table 1 below. Benralizumab (Fasenra®) Dupilumab (Dupixent®) Mepolizumab (Nucala®) Omalizumab (Xolair®) Reslizumab (Cinqair®) GENERAL CRITERIA FOR INITIAL PRIOR AUTHORIZATION: (must meet all of the following) • Must be approved for the indication, age, and not exceed dosing limits listed in Table 1. • Must be prescribed by or in consultation with a pulmonologist, allergist, or immunologist.1,2 • For all agents listed, the preferred PDL drug, which treats the PA indication, is required unless the patient meets the non-preferred PDL PA criteria. • Must have experienced ≥ 2 exacerbations within the last 12 months despite meeting all of the following (exacerbation is defined as requiring the use of oral/systemic corticosteroids, urgent care/hospital admission, or intubation: o Patient adherence to two long-term controller medications, including a high-dose inhaled corticosteroid 1,2 (ICS) and a long-acting beta2-agonist (LABA) listed in Table 2. ▪ Combination ICS/LABA and ICS/LABA/LAMA products meet the requirement of two controller medications. o Patient must have had an adequate trial (at least 90 consecutive days) of a leukotriene modifier or a long-acting muscarinic antagonist (LAMA) as a third long-term controller medication listed in Table 2. -

Inadequate Assessment of Adherence To€Maintenance Medication Leads
ORIGINAL ARTICLE ASTHMA Inadequate assessment of adherence to maintenance medication leads to loss of power and increased costs in trials of severe asthma therapy: results from a systematic literature review and modelling study Matshediso C. Mokoka1, Melissa J. McDonnell2, Elaine MacHale1, Breda Cushen1, Fiona Boland3, Sarah Cormican2, Christina Doherty4, Frank Doyle 5, Richard W. Costello6 and Garrett Greene1 @ERSpublications Trials of add-on therapy for severe asthma do not include adequate assessment of adherence to maintenance therapy. As a result, they suffer from a significant loss of statistical power, leading to greatly increased sample sizes and associated costs. http://ow.ly/ZCyH30nSFHc Cite this article as: Mokoka MC, McDonnell MJ, MacHale E, et al. Inadequate assessment of adherence to maintenance medication leads to loss of power and increased costs in trials of severe asthma therapy: results from a systematic literature review and modelling study. Eur Respir J 2019; 53: 1802161 [https://doi. org/10.1183/13993003.02161-2018]. ABSTRACT Adherence to inhaled maintenance therapy in severe asthma is rarely adequately assessed, and its influence on trial outcomes is unknown. We systematically determined how adherence to maintenance therapy is assessed in clinical trials of “add-on” therapy for severe asthma. We model the improvement in trial power that could be achieved by accurately assessing adherence. A systematic search of six major databases identified randomised trials of add-on therapy for severe asthma. The relationship between measuring adherence and study outcomes was assessed. An estimate of potential improvements in statistical power and sample size was derived using digitally recorded adherence trial data. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

Dupixent (Dupilumab) Fasenra (Benralizumab) Nucala (Mepolizumab) Xolair (Omalizumab) Effective January 1, 2021
Asthma and Allergy Injectables Cinqair (reslizumab) Dupixent (dupilumab) Fasenra (benralizumab) Nucala (mepolizumab) Xolair (omalizumab) Effective January 1, 2021 ☐ MassHealth Plan ☒ ☒Commercial/Exchange Prior Authorization Program Type ☐ Quantity Limit ☒ Pharmacy Benefit Benefit ☐ Step Therapy ☒ Medical Benefit (NLX) Specialty This medication has been designated specialty and must be filled at a contracted Limitations specialty pharmacy when obtained through the pharmacy benefit. Specialty Medications All Plans Phone: 866-814-5506 Fax: 866-249-6155 Non-Specialty Medications Contact MassHealth Phone: 877-433-7643 Fax: 866-255-7569 Information Commercial Phone: 800-294-5979 Fax: 888-836-0730 Exchange Phone: 855-582-2022 Fax: 855-245-2134 Medical Specialty Medications (NLX) All Plans Phone: 844-345-2803 Fax: 844-851-0882 Cinqair, Fasenra, Nucala and Xolair solutions are Medical Benefit only Exceptions Dupixent, Fasenra Pen, Nucala Pen and Xolair Pen are Pharmacy Benefit Only and obtained through specialty pharmacy Overview Cinqair and Fasenra are interleukin-5 antagonist monoclonal antibodies indicated for: • As add-on maintenance treatment of severe asthma for members with an eosinophilic phenotype. Nucala is an interleukin-5 antagonist monoclonal antibody indicated for: • Treatment of severe asthma with an eosinophilic phenotype • Eosinophilic granulomatosis with polyangiitis • Hypereosinophilic syndrome (HES) Dupixent is an interleukin-4 receptor alpha agonist indicated for: • Atopic Dermatitis • Chronis rhinosinusitis with nasal polyps -

Samaritan Fund
Items supported by the Samaritan Fund (a) Non-drug Items supported by the Fund (b) Other items supported by the Samaritan Fund Mechanism (c) Self-financed Drugs supported by the Samaritan Fund (SF) and Community Care Fund (CCF) Medical Assistance Programme (First Phase Programme) (for specified self- financed cancer drugs) (a) Non-drug Items supported by the Fund 1. Percutaneous Transluminal Coronary Angioplasty (PTCA) and other consumables for interventional cardiology 2. Cardiac Pacemakers 3. Myoelectric Prosthesis 4. Custom-made Prosthesis 5. Appliances for prosthetic and orthotic services, physiotherapy and occupational therapy services (e.g. prosthesis) 6. Home use equipment and appliances (e.g. wheelchair, replacement of external speech processor for patients done with cochlear implant) 7. Gamma knife surgery 8. Harvesting of marrow in a foreign country for marrow transplant The Fund will only support the model which can meet the basic medical needs of the patients. (b) Other items supported by the Samaritan Fund Mechanism 1. Positron Emission Tomography (PET) service (c) Drugs supported by the Samaritan Fund The following specific self-financed drugs are supported by the Samaritan Fund: Item Drug Types of Clinical indications diseases 1 Abatacept Rheumatology Rheumatoid arthritis 2a Adalimumab Dermatology Severe psoriasis 2b Ophthalmology Non-infectious intermediate, posterior and panuveitis 2c Paediatric chronic non-infectious anterior uveitis 2d Rheumatology Ankylosing spondylitis 2e Juvenile idiopathic arthritis 2f Psoriatic -

EOSINOPHIL DEPLETION by ANTIBODY TREATMENT: Lessons Learned from Clinical Trials
EOSINOPHIL DEPLETION BY ANTIBODY TREATMENT: Lessons Learned from Clinical Trials Amy Klion, MD Laboratory of Parasitic Diseases February 26, 2013 Potential Targets for Eosinophil Depletion CD52 (alemtuzumab) Common β chain GM-CSF IL-2R (daclizumab) IL-4 IL-4R IL-9 IL-13 IL-31 IgE (omalizumab) TSLP (Wechsler et al. 2012 JACI) Antibodies in development: IL-5/IL-5R Mepolizumab Reslizumab Benralizumab (SB-240563) (SCH55700) (MEDI-563) Target IL-5 IL-5 IL-5Rα Antibody Humanized IgG1κ Humanized IgG4κ Humanized (parent) (murine 2B6) (rat 39D10) afucosylated IgG1κ Kd 4.2 pmol/L 20 pmol/L 26 pmol/L t1/2 20 days 30 days 16 days Max dose in 10 mg/kg iv 3 mg/kg iv 3 mg/kg iv human trials 250 mg sc 200 mg sc Status Phase III ongoing Phase III ongoing Phase II completed Assumptions • Eosinophils are responsible for the clinical manifestations of the disorder • Blood and tissue eosinophils are equivalent in their responses to antibody therapy • The clinical efficacy endpoint is appropriate for the disorder being studied Asthma and Eosinophils: 1990-2000 (Barnes JACI 1989) (Busse NEJM 2000) Asthma and anti-IL-5 antibody: early trials • Leckie et al. Lancet • Kips et al. Am J Resp Crit 2000 Care Med 2003 • Double-blind, placebo- • Double-blind, placebo- controlled, single dose trial controlled, single dose of mepolizumab escalation trial of reslizumab (2.5 or 10 mg/kg iv) (0.03-1 mg/kg iv) • Study population: mild • Study population: severe allergic asthma (n=24) persistent asthma (n=32) • Efficacy endpoints: • Efficacy endpoints: • late asthmatic response ✘ • FEV1 ✘ (drop in FEV1 at 4-10 hrs) ✔ • blood eosinophilia to allergen challenge ✘ • sputum eosinophilia ✔ • blood eosinophilia ✘ • symptom severity ✔ • sputum eosinophilia Why did these early trials fail? Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions Role of eos in asthma? Buttner et al. -
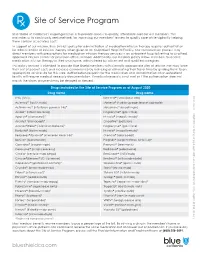
Site of Service Program
Site of Service Program Blue Shield of California’s ongoing mission is to provide access to quality, affordable care for our members. This motivates us to continually seek methods for improving our members’ access to quality care while vigilantly helping them contain associated costs. In support of our mission, Blue Shield’s policy for administration of medication infusion therapy requires authorization for administration of infusion therapy when given at an Outpatient Hospital Facility. Our authorization process may direct members with prescriptions for medication infusion therapy services in an outpatient hospital setting to qualified, approved infusion centers or physician offices instead. Additionally, our medical policy allows members to receive medication infusion therapy in their own home, administered by a licensed and qualified caregiver. This policy revision is intended to provide Blue Shield members with clinically appropriate sites of service that may lower their out-of-pocket costs and increase convenience by reducing or eliminating their travel time by guiding them to an appropriate service site for this care. Authorization requests for the medication and administration at an outpatient facility will require medical necessity documentation. If medical necessity is not met or if the authorization does not match the claim, payment may be delayed or denied. Drugs included in the Site of Service Program as of August 2020 Drug name Drug name IVIG (IVIG) Kanuma® (sebelipase alfa) Actemra® (toclizumab) Makena® (hydroxyprogesterone -

Batch 55 Block Scoping Report
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE CENTRE FOR HEALTH TECHNOLOGY EVALUATION Technology Appraisals Consultation on Batch 55 draft remits and draft scopes and summary of comments and discussions at scoping workshops Topic ID Topic title 1197 Liraglutide for preventing cardiovascular events in people with type 2 diabetes 1158 Ertugliflozin for treating type 2 diabetes 1189 Naldemedine for treating opioid-induced constipation 1194 Ixekizumab for treating active psoriatic arthritis following inadequate response to disease-modifying anti-rheumatic drugs 1198 Eltrombopag for untreated severe aplastic anaemia 1186 Mepolizumab for treating eosinophilic granulomatosis with polyangiitis 1185 Caplacizumab for treating acute acquired thrombotic thrombocytopenic purpura 1188 Erenumab for preventing migraine 1060 Tildrakizumab for treating moderate to severe plaque psoriasis TA Block scoping report – Batch 55 September 2017 Page 1 of 16 Commercial in confidence information removed © National Institute for Health and Care Excellence 2017 All rights reserved Liraglutide for preventing cardiovascular events in people with Provisional Title type 2 diabetes Topic Selection 8967 Wave / Round R227 ID Number TA ID Number 1197 Company Novo Nordisk Anticipated licensing ***CONFIDENTIAL INFORMATION REMOVED*** information To appraise the clinical and cost effectiveness of liraglutide Draft remit within its marketing authorisation for preventing cardiovascular events in people with type 2 diabetes. Following the consultation exercise, NICE is of the opinion that an appraisal of liraglutide monotherapy for preventing cardiovascular events is appropriate. A new referral is not sought because the remit for TA203 also covers this indication. Some stakeholders highlighted that the appraisal is urgent as it would give more people at high risk of CVD another therapy to reduce their risk of CVD events. -

Soluble Ligands As Drug Targets
REVIEWS Soluble ligands as drug targets Misty M. Attwood 1, Jörgen Jonsson1, Mathias Rask- Andersen 2 and Helgi B. Schiöth 1,3 ✉ Abstract | Historically, the main classes of drug targets have been receptors, enzymes, ion channels and transporters. However, owing largely to the rise of antibody- based therapies in the past two decades, soluble protein ligands such as inflammatory cytokines have become an increasingly important class of drug targets. In this Review, we analyse drugs targeting ligands that have reached clinical development at some point since 1992. We identify 291 drugs that target 99 unique ligands, and we discuss trends in the characteristics of the ligands, drugs and indications for which they have been tested. In the last 5 years, the number of ligand-targeting drugs approved by the FDA has doubled to 34, while the number of clinically validated ligand targets has doubled to 22. Cytokines and growth factors are the predominant types of targeted ligands (70%), and inflammation and autoimmune disorders, cancer and ophthalmological diseases are the top therapeutic areas for both approved agents and agents in clinical studies, reflecting the central role of cytokine and/or growth factor pathways in such diseases. Drug targets In the twentieth century, drug discovery largely involved far more challenging to achieve with small- molecule Pharmacological targets, such the identification of small molecules that exert their drugs. Protein ligands have been successfully targeted as proteins, that mediate the therapeutic effects by interacting with the binding sites by many drugs since the first FDA approval of the desired therapeutic effect of of endogenous small- molecule ligands such as neuro- ligand- targeting agents etanercept and infliximab in a drug. -

IL-5 ANTAGONISTS Fasenra (Benralizumab) Nucala (Mepolizumab)
IL-5 ANTAGONISTS Fasenra (benralizumab) Nucala (mepolizumab) RATIONALE FOR INCLUSION IN PA PROGRAM Background Fasenra (benralizumab) and Nucala (mepolizumab) are used with other asthma medications for the maintenance treatment of asthma in patients with an eosinophilic phenotype. Fasenra and Nucala are approved for patients who have a history of severe asthma attacks (exacerbations) despite receiving their current asthma medicines. Fasenra and Nucala reduce severe asthma attacks by reducing the levels of blood eosinophils- a type of white blood cell that contributes to the development of asthma. Nucala is also used in the treatment of certain eosinophilic conditions and in chronic rhinosinusitis with nasal polyps (CRSwNP) (1-2). Regulatory Status FDA-approved indication: Fasenra is interleukin-5 antagonist monoclonal antibodies (IgG1 kappa) indicated for add-on maintenance treatment of patients with severe asthma ages 12 years and older, and with an eosinophilic phenotype (2). Nucala is an interleukin-5 antagonist monoclonal antibody (IgG1 kappa) indicated for: (1) 1. Add-on maintenance treatment of patients with severe asthma aged 6 years and older, and with an eosinophilic phenotype 2. Add-on maintenance treatment of adult patients 18 years and older with chronic rhinosinusitis with nasal polyps (CRSwNP) with inadequate response to nasal corticosteroids 3. The treatment of adult patients with eosinophilic granulomatosis with polyangiitis (EGPA) 4. The treatment of adult and pediatric patients aged 12 years and older with hypereosinophilic