Water Analysis Is Essential for Potable Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Village Level Localbodies of BERASIA(Janpad Panchayat),BHOPAL(Zila Panchayat),MADHYA PRADESH *N.A.- Notapplicable
7/18/2019 Village Level Localbodies of BERASIA(Janpad Panchayat),BHOPAL(Zila Panchayat),MADHYA PRADESH *N.A.- NotApplicable Sr Local Body Local Body Name (In No. of Villages Local Body Name Local Body Type Map No. Code Local language) Mapped 1 134252 AMARPUR Gram Panchayat 2 2 134253 ANKIA Gram Panchayat 4 3 134254 ARJUNKHEDI Gram Panchayat 4 4 134255 ARRAWATI Gram Panchayat 3 5 134256 BABACHIYA Gram Panchayat 1 6 134257 BADBELI KALAN Gram Panchayat 2 7 134258 BAGSI Gram Panchayat 2 8 134259 BAHRAWAL Gram Panchayat 3 9 134260 BAIRAGARH Gram Panchayat 3 10 134261 BANDARUA Gram Panchayat 7 BARKHEDA 11 134262 Gram Panchayat 2 BARAMAD 12 134263 BARKHEDA YAKUB Gram Panchayat 2 13 134264 BARODI Gram Panchayat 3 14 134265 BARRAI Gram Panchayat 5 BARRICHHEER 15 134266 Gram Panchayat 2 KHEDA 16 134267 BARRIE BAGRAJ Gram Panchayat 5 17 134268 BEELKHON Gram Panchayat 2 18 236195 BHAINSKHEDA Gram Panchayat 2 19 134269 BHAISONDA Gram Panchayat 1 20 134270 BHUJPURKALAN Gram Panchayat 3 21 134271 BIRHASHYAMKHEDI Gram Panchayat 4 22 134272 CHANDA SALOI Gram Panchayat 5 23 134273 CHATAHEDI Gram Panchayat 2 24 134275 DAMILA Gram Panchayat 7 25 134274 DAM KHEDA Gram Panchayat 1 26 134282 DAULATPURA Gram Panchayat 4 27 134276 DEVALKHEDA Gram Panchayat 4 28 134277 DHAMARRA Gram Panchayat 1 29 134278 DHATURIYA Gram Panchayat 4 30 134279 DHOOT KHEDI Gram Panchayat 2 31 134280 DILLOD Gram Panchayat 1 32 134281 DOHAYA Gram Panchayat 3 33 134283 DUNGARIYA Gram Panchayat 1 34 236196 GANGAPIPALIYA Gram Panchayat 1 35 134284 GARENTHIYADANGI Gram Panchayat 3 36 134285 GARHA KALAN Gram Panchayat 1 37 134286 GARHA KHURD Gram Panchayat 4 38 134287 GUJARTODI Gram Panchayat 7 39 134288 GUNGA Gram Panchayat 1 40 134289 HABIBGANJ Gram Panchayat 1 1/3 7/18/2019 Sr Local Body Local Body Name (In No. -

District Census Handbook, Bhopal, Part XIII-B, Series-11
"lif XIII -. 'fiT • • ~. ,,1.1-, "T1;cft~ 5I"lImrfif'li 6~J f;{~w", ~;:rqwr;:rr 'itA!' sr~1!f 1981 CENSUS-PUBLICATION PLAN (1981 Cemuv Pub!icatil')m, Series 11 Tn All India Series will be pu!J/is1led ill '!le fJllowing PlJl'1s) GOVERNMENT OF INDIA PUBLiCATIONS Part I-A Administration Report-Enumeration Part I-B Administration Report-Tabulation Part I1.~ General Population Tables Part II-B Primary Census Abstract Part III General Economic Tables Part IV Social and Cultural Tables Part V Migration Tables Part VI Fertility Tables Part VJI Tables on Houses and Diiabled PopulatioD Part VIII llousehold Tables Part IX SJX:cial Tables on Scheduled Castes and Scheduled Tribes Part X-A Town Directory Part x-B Survey Reporti on 5elected Towns Part X-C Survey Reports on selected Villages Part XI Ethnographic Notes and special studie. on Scheduled Castel and Scheduled Tribes Part XII Census Atlas Paper I of 1982 Primary Census Abstra~t for Sc!1eduled Castes and Schedul cd Tribes Paper 1 of 1984 Household Population by Religion of Head or Hou':lehold STATE GOVERNMENT PUBLICATIONS Part XIII-A&B District CetlslIs H:mdbook for each of the 45 districts in the State. (Village and Town Directory and Primary Census Abstract) comE~TS T'O Pages Foreword i-iv Preface v-vi District Map I'llportant Statistics vii Analytical Note ix-xxxiv ~lell'Tl'~lIi fecq-urr, 81'~~f:qa ;snfa 81"h: ari!~t~i.'I' Notes & Explanations, List of Scheduled Castes and Sched uled Tribes Order iil'rr~Tfo Off ~ifr (<<w)arr), mTl1ifi 1976, (Amendment) Act. -
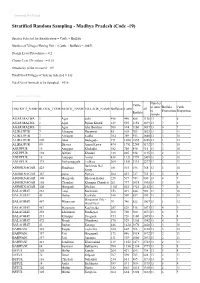
Stratified Random Sampling - Madhya Pradesh (Code -19)
Download The Result Stratified Random Sampling - Madhya Pradesh (Code -19) Species Selected for Stratification = Cattle + Buffalo Number of Villages Having 500 + (Cattle + Buffalo) = 20453 Design Level Prevalence = 0.2 Cluster Level Prevalence = 0.01 Sensitivity of the test used = 0.9 Total No of Villages (Clusters) Selected = 332 Total No of Animals to be Sampled = 4316 Back to Calculation Number Cattle of units Buffalo Cattle DISTRICT_NAME BLOCK_CODE BLOCK_NAME VILLAGE_NAME Buffaloes Cattle + all to Proportion Proportion Buffalo sample AGAR MALWA 1 Agar Salri 448 440 888 1136 13 7 6 AGAR MALWA 1 Agar Piplon Khurd 619 535 1154 1899 13 7 6 AGAR MALWA 1 Agar Ahir Bardiya 506 674 1180 1437 13 6 7 ALIRAJPUR 7 Alirajpur Haraswat 85 618 703 1823 13 2 11 ALIRAJPUR 7 Alirajpur Kodla 184 769 953 2680 13 3 10 ALIRAJPUR 209 Jobat Badaguda 171 1684 1855 4258 13 1 12 ALIRAJPUR 80 Bhavra Aman Kuwa 494 1751 2245 5192 13 3 10 ANUPPUR 18 Anuppur Kholadhi 182 708 890 915 13 3 10 ANUPPUR 194 Jaithari Khodari 133 805 938 1135 13 2 11 ANUPPUR 18 Anuppur Amlai 438 1321 1759 2009 13 3 10 ANUPPUR 375 Pushparajgarh Ledhara 264 1851 2115 2277 13 2 11 Barkheda Nai ASHOKNAGAR 425 Shadhora 181 515 696 768 13 3 10 Saray ASHOKNAGAR 367 piprai Pipriya 304 423 727 735 13 5 8 ASHOKNAGAR 308 Mungaoli Bhaison Kalan 228 519 747 830 13 4 9 ASHOKNAGAR 105 Chanderi Khanpur Chanderi 261 777 1038 1405 13 3 10 ASHOKNAGAR 308 Mungaoli Dhekan 1103 822 1925 2142 13 7 6 BALAGHAT 261 Lanji Borikalan 153 491 644 989 13 3 10 BALAGHAT 43 Baihar Karwahi 148 509 657 890 13 3 10 Waraseoni -

District Census Handbook, Bhopal, Part XIII-A, Series-11
'11tT XIII-CR ~'I~'Co 11 • • 1981 C.~NSUS-PUBLICATION PLAN (1981 Celtrll> Publicati'JllY, Series 11 in AlII"dl:J Series wIll be publislted in the following Prl,uts) GOVERNMENT OF INDIA PUBLICA nONS Part I-A Administration Report-Enumeration Part I·B Administration Report-Tabulation Part II·A General Population Tables Part II·B Primary Census Abstract Part III General Economic Tables Part IV Social and Cultural Tables Part V Migration Tables Part VI Fertility Tab!es Part VII Tables on Houses and Disabled Population Part VIII Household Tahles Part IX Special Tables on Scheduled Castes and Scheduled Tribes Part X-A Town Directory Part X-B Survey Report. on selected Towns Part X-C Survey Reports on selected Villages Part XI Ethnographic Notes and special studies on :scheduled Castes and Scheduled Tribes Part XII Census Atlas Paper I -of 1982 Primary Census Abstract for Scheduled Castes and Scheduf ed Tribes Paper 1 of 1984 Household Population by Religion of Head of Houo;ehold STATE GOVERNMENT PUBLICATIONS Part XIII-A&B District Census Handbook for each of the 45 districts in the State. (Village and Town Directory and Primary Census Abstract) CONTENTS crto.. ij'~lfT Pages 1 ~'f;r Foreword ;-iv 2 ~'(Cf"fT Preface v-vi 3 f~ ~ if1.f!ll'T Distri(;t Map 4 if~~:Jf~ artCfi~ Important Statistics vii 5 fat1lilrq1Jf'f~ifi {?;cqvr"t Analytical Note xxi-xxxiv ~Cififi RCtJurT : ar2;~m iiIlfff ait~ ar.t~a Notes & Explanations; List of Scheduled Gf"f"TTrn- Ofil' ~T. ( ~w)F1;r ), mlfi 1 976; Castes and Scheduled Tribes Order ~T iif'l'lTur;;T 9;ila-IfiT !fiT ~fuijJ~ ar1~ ~~ I (Amendment) Act, 1976. -

District Census Handbook, Bhopal, Part XII-A & B, Series-13
q~clT-13 SEI~IES - 13 ~~~ MADI-IYA, PRADESlI Ri1 (lil "VFP I 0 I "11 ~ R=ct Cf} I DIS'I'I<lCT CENSUS I-Ip,.ND BOOK 'l-Wf XII -. Cf) ~ ~. PART XII - A <-f?:._ B ,-. r-.... ! \J1 ('11 \iJ;1'\ i U i "i I ":11'{\1 q)1 (7Jp:f q ~.rrx ~Frfucpl· ~-jlh' lJP"f \;<i -::rrIit l)T~ 'JFfTfuAr ~HH) DISTRlC1_-' C=ENSUS HAN-D BOOK \TILLAGE &: T()\VN DIREC'rORY AND VILLA.(;E '-~ TOWN PRIlVIARY CENSUS ABS1~Rj\.CT ~ q I cl -Rilc;rr BHOPAL DISTRICT ~T <nl<f 1~ ~ II C"t4. ~"i!"~ - DIRECTORATE OF CENSUS OPERATIO~S, IVIADHYA PRADESH Foldcr-f'n.:ss r: ik-Blwpal- I c I ~-~~ CONTENTS ~ Pages ~ ••..•••••••.......•.•.••••••••.•••.••••..••.•••••••••••••••••••••..•.••••••• V Foreword .•.••..•...••....••••.•••.•••••..•.•••.•••••••••••••••••••••••••••.••••• • .• VI I'H''fIlcFll ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• VII Preface ••••••••••••..•• : ........................................................... VII I ~ .•....•.•..............•.....................•....•.....: ...................... IX Acknowledgement • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • •• • ••••••••• X ~ cnr rrcmT Map of the District •...............•.....................•....•............•.. .- ........Xl 'ii5M 'l0j ~ •...•................•.•.......••..••••.•..•.••.•.......•....•..•••.. '1-3 Important Statistics ~ \[<P ~ 'it ................ ~ . • . • . •. 4-1 9 Figures at a Glance fi:cqfOllti' C1W ~ •••• ". •••••••••••••••••••••••••••••••••••••••••••••••••••••••••• 21-29 Notes and Explanations -

PIN CODES in District Bhopal (MP) Village/Locality Name Officename ( BO/SO/HO) Pincode Sub-Distname Huzur Jumerati S.O 462001 Huzur Huzur S.I
PIN CODES in District Bhopal (MP) Village/Locality name Officename ( BO/SO/HO) Pincode Sub-distname Huzur Jumerati S.O 462001 Huzur Huzur S.I. Line S.O 462001 Huzur Huzur Chhola Road S.O 462001 Huzur Huzur Hamidia Road S.O 462001 Huzur Huzur Berasia Road S.O 462001 Huzur Huzur Old Secretriate S.O 462001 Huzur Huzur Shahajahanabad S.O 462001 Huzur Huzur Bhopal G.P.O. 462001 Huzur Huzur Gandhi Medical College S.O 462001 Huzur Huzur Itwara S.O 462001 Huzur Huzur Regional College S.O 462002 Huzur Huzur Vidya Vihar S.O 462002 Huzur Huzur C.T.T.Nagar H.O 462003 Huzur Huzur Shastri Nagar S.O (Bhopal) 462003 Huzur Huzur Raj Bhawan S.O (Bhopal) 462003 Huzur Huzur Tulsi Nagar S.O 462003 Huzur Huzur Kamla Nagar S.O (Bhopal) 462003 Huzur Huzur M.A.C.T. S.O 462003 Huzur Huzur Satpura S.O 462004 Huzur Huzur M.P. Vidhan Sabha S.O 462004 Huzur Huzur Vallabh Bhawan S.O 462004 Huzur Huzur Barkhedi S.O 462008 Huzur Huzur Jahangirabad S.O (Bhopal) 462008 Huzur Garhmurra Sukhi Sewania B.O 462010 Huzur Gaushala Sukhi Sewania B.O 462010 Huzur Huzur Chandbad S.O 462010 Huzur Kalyanpur Pipaliya Jaheerpeer B.O 462010 Huzur Huzur Sikandri Sarai S.O 462010 Huzur Mavaishi Bazar Imaliya B.O 462010 Huzur Rajgarh Imaliya B.O 462010 Huzur Bansiya Pipaliya Jaheerpeer B.O 462010 Huzur Bhadbhada Chowki Sukhi Sewania B.O 462010 Huzur Chohrai Balampur B.O 462010 Huzur Pipaliya Rani Imaliya B.O 462010 Huzur Rasla Khedi Imaliya B.O 462010 Huzur Balampur Balampur B.O 462010 Huzur Ghat Khedi Imaliya B.O 462010 Huzur Mahua Kheda Balampur B.O 462010 Huzur Meerpur Pipaliya Jaheerpeer -

LOWER DIVISION CLERK (POST CODE-02) NAME of the FATHER's/ Sno APPLICANT HUSBAND's NAME DOB CAT
LIST OF CANDIDATES TO BE CALLED FOR WRITTEN EXAMINATION ON 17.08.2014 (SUNDAY) FOR THE POST OF LOWER DIVISION CLERK (POST CODE-02) NAME OF THE FATHER'S/ Sno APPLICANT HUSBAND'S NAME DOB CAT. PRESENT ADDRESS H.NO. 9-7-173/1, EAST MARUTI NAGAR A. PRAVEEN (OPP):- SANTOSH NAGAR, CHAMPAPET, 1 KUMAR CHANDRU 13.04.1992 ST HYDERABAD - 500079 ASHOK KUMAR 283/2A MUNIRKAVILLAGE, 2 A. PRIYA RAI RAI 23.04.1995 UR NEW DELHI- 110067 NO. -37 A, FRIST STREET PERIA KASI KOVIL KUPPAN NEAR PERANAL HEALTH CARE 3 A.LEELAVATHI N.ARUMUGAM 02.07.1986 OBC ENNORE, CHENNAI -600057 B II- 544 RAGHUBIR NAGAR 4 AAKANKSHA ANIL KUMAR 28.9.1991 UR NEW DELHI -110027 SHIVAM VASTRALAY AAKANKSHA KAMTA PRASAD GOHPARU SHANDOL, MADHYA PRADESH- 5 SONI SONI 10.07.1992 OBC 484770 B-27 UPPER GROUND FLOOR OLD GOBIND AAKANKSHA PURA (NEAR BALDEV PARK) NEW DELHI - 6 SRIVASTAV R. K. SRIVASTAVA 28.08.1988 UR 110051 H. NO. A-706 GALI NO. 14 PRATAP NAGAR 7 AAKASH KUMAR RAKESH KUMAR 29.05.1992 SC SABOLI DELHI -110093 MOH. -LOTANPUR MANDIR WALI GALI 8 AAKASH ROHELE KHAJANCHI ROHELE 28.03.1989 SC BUDAUN, UTTAR PRADESH -243601 A-833 MANGOLPURI, 9 AAKASH SINGH VIRENDER SINGH 03.06.1989 ST NEW DELHI- 110083 RAJ KUMAR D1/218 GALI NO.- 11, AMBEDKAR MARG, 10 AAKRITI GAUTAM 05.11.1992 SC HARSH VIHAR, SAHADARA DELHI -110093 CHARANJEET 539 SECTOR-15A FARIDABAD HARYANA - 11 AAKRITI CHUGH CHUGH 30.08.1994 UR 121007 AANOUKSHA I. R. SOMESH WZ/G-56 UTTAM NAGR NEAR MCD PRIMARY 12 GOSWAMI GOSWAMI 15.03.1995 UR SCHOOL NEW DELHI -110059 WARD NO.-08, CENTRAL SCHOOL ROAD BHARWELI, DISTT. -

Water Pollution Minimize to Protect the Life of Human Being
International Journal of Science and Research (IJSR) ISSN: 2319-7064 ResearchGate Impact Factor (2018): 0.28 | SJIF (2018): 7.426 Water Pollution Minimize to Protect the Life of Human Being Dr. Ratna Roy (Pathak) Professor of Chemistry, Govt. M.L.B. Girls PG Autonomous College Bhopal M.P. India Abstract: Water pollution is one of the most serious problems facing humanity and other life forms on our planet today. Water pollution is defined as the contamination of the physical and biological component of earth system to such an extent that normal environmental processes are adversely affected! Pollutant can be naturally occurring substances or energies but they are considered contaminant in excess of natural level. Any use of natural resources at a rate higher than nature's capacity to restore itself can result in pollution of air, water and land. Pollution prevention is a major global concern because of the harmful effects of pollution on a person’s health and on the environment. Environmental pollution comes in various forms, such as: air pollution, water pollution, soil pollution, etc. Everyone is a stakeholder as we are all inhabitants of this one and only mother earth. Each person can contribute something to advance environmental pollution mitigation measures. Environmental protection means caring for our resources and subsequently for ourselves and ensuring a sustainable future for generations to come will have a better environment. You and I should therefore accept personal responsibility for the success of the environmental protection programs of our respective community by cooperating and actively participating in making the atmosphere pollution free. -

Sub Center List
S.No Districts Blocks Ø-la- Name of midsUnzks dk uke Sub Center 1 Alwar Bansur 1 Girudi fx:Mh (m½ 2 Alwar Bansur 2 Lekdi ysdMh (m½ 3 Alwar Bansur 3 Bhoopseda HkwilsMk (m½ 4 Alwar Bansur 4 Kheda [ksMk (m½ 5 Alwar Bansur 5 Ladpura ykMiqjk 6 Alwar Bansur 6 Mothuka eksBwdk 7 Alwar Bansur 7 Buteri cqVsjh 8 Alwar Bansur 8 Sanpura lkuiqjk 9 Alwar Bansur 9 Unchpur mWapiqj 10 Alwar Bansur 10 Choola pwyk 11 Alwar Bansur 11 Lalpura ykyiqjk 12 Alwar Bansur 12 Babria ckcfj;k 13 Alwar Bansur 13 Morodi (95-96) eksjksMh (95&96½ 14 Alwar Bansur 14 Chatarpura prjiqjk 15 Alwar Bansur 15 Bilali fcykyh 16 Alwar Bansur 16 Mahanpur eguiqj 17 Alwar Bansur 17 Baberi ccsjh 18 Alwar Bansur 18 Bilath fcykB 19 Alwar Bansur 19 Kishorpura fd'kksjiqjk 20 Alwar Bansur 20 Chandali pUnkyh 21 Alwar Bansur 21 Jhanjharpur >a>kjiqjk 22 Alwar Bansur 22 Kalayan nagar (96-97) dY;k.k uxj (96&97½ 23 Alwar Bansur 23 Aalampur (T) vkyeiqj (LFkk-½ 24 Alwar Bansur 24 Dangiwas Mkaxhokl 25 Alwar Bansur 25 Sainthalpur lasFkyiqj 26 Alwar Bansur 26 GYampur (96-97) x;keiqj (96&97½ 27 Alwar Bansur 27 Bhagu ka baas (96-97) Hkkxw dk okl (96&97½ 28 Alwar Bansur 28 Devsan (96-97) nsolu (96&97½ 29 Alwar Bansur 29 Nangal bhavsingh (96-97) ukaxy Hkkoflag (96&97½ 30 Alwar Bansur 30 Khori (96-97) [kksgjh (96&97½ 31 Alwar Bansur 31 Bas shekhawat (96-97) ckl 'ks[kkor (96&97½ 32 Alwar Bansur 32 Kanpur Kanjipura (97-98) dkuiqjk (dkuthiqjk½ (97&98½ 33 Alwar Bansur 33 Shahpura (97-98) kkgiqjk (97&98½ 34 Alwar Bansur 34 Purohit ka baas (97-98 T) iqjksfgr dk ckl (97&98½ (LFkk-½ 35 Alwar Bansur 35 Baman vaas -

District Census Handbook, Sehore, Parts X (A) &
CENSUS OF INDIA 1971 SERIES 10 MADHYA PRADESH DISTRICT CENSUS ~:aOOK PARTS X(A) & X(B) VILLAGE AND TOWN DIRECTORY VILLAGE AND TOWN-WISE PRIMARY CENSUS ABSTRACT SEHORE DISTRICT A. K. PANDYA ;>'\ __ : ,,-,; - '~.' . OF THE INDIAN ADMINisTRATIVE SERViCE DIRECTOR OF CENSUS OPERATIONS, MADHYA pRADESH PUBLISHED BY THE OOVT. OF MADHVA PRADESH 1975 1971 CENSUS PUBUCA110NS, MAl>HYA PRADESH (AD the Census PublicatiGDs of this State will bear series No. 10) PART I Census General Report including Subsidiary (in Sub-Parts) Tables. PART II-A Census Tables on population. PART II-B Economic Tables. (in Sub-Parts) PART II-C Social and Cultural Tables. (in Sub-Parts) PART III-A Establishment Report and Subsidiary Tables. PART III-B Establishmenl Tables. PART IV Housing Report and Tables. PART V (in Sub-Parts) Special T~bles & Ethnographic Notes OD Scheduled Castes & Scheduled Tribes. PART VI-A Town Directory. PART VI-B Special Survey Reports on selected Towns. , , PART VI-C , SurVey Reports: on selected Villages. PART VII . Special Report on Graduates and Technical Personnel. PART VIII-A Administration Report-Enumeration. PART VIII-B Administration Report-Tabulation. PART IX Census Atlas. PART IX-A .. Ad~nistrative Atlas. STATE GOVERNMENT PUBUCATIONS PART X-A Village and Town Directory PART X-B Village and Town Primary Census Abstracts PART X-C .. Analytical Report and Administrative statements & District Census Tables (District Census Handbooks art published under Part .• In aParts-A, B & C for each of the 43 districts in 'Iw ,state. Parts A and B are published in one volume). CONTENTS Page 1. Preface i-ii 2. -

Berasia Assembly Madhya Pradesh Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781, 26810964-65-66 Email : [email protected] Website : http://www.datanetindia-ebooks.com Report No. : AFB/MP-149-0617 ISBN : 978-93-87165-16-8 First Edition : June, 2017 Third Updated Edition : June, 2019 Price : Rs. 11500/- US$ 310 © 2017 Datanet India Pvt. Ltd. All right reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. Printed in India No. Particulars Page No. Introduction 1 Assembly Constituency at a Glance | Features of Assembly as per 1-2 Delimitation Commission of India (2008) Location and Political Maps 2 Location Map | Boundaries of Assembly Constituency in District | Boundaries 3-9 of Assembly Constituency under Parliamentary Constituency | Town & Village-wise Winner Parties- 2014, 2013, 2009 and 2008 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 10-28 Villages | Village Panchayat | Intermediate Panchayat Demographic 4 Population Households | Rural/Urban Population | Towns and Villages by 29-30 Population Size | Sex Ratio (Total & 0-6 Years) | Religious Population | Social Population | Literacy Rate | Work Participation Electoral Features Important Dates of Last Elections