Newsletter of the Pineapple Working Group, International Society for Horticultural Science ______Issue No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transfer of UGT/AT(Pry)/KBT/CT/GT of Dhalai District Under
No.F. 1(2- 1)-DEE/EST| 12017 (L-76) Government of Tripura Directorate of Elementary Education (Esstablishment Section) Dated, AgartaTa,l2- lL/ zotz. MEMO. Subject: - Transfer of Under Graduate Teacher/Assistant Teacher (Pry)/Kok-Borok Teacher/ Contractual Teacher/Graduate Teachers. In Public interest, 72 (Seventy Two) Nos. Under Graduate Teacher/Assistant Teacher (Pry)/Kok-Borok Teacher/ Contractual Teacher / Graduate Teachers as per list enclosed are hereby transferred from their existing places of posting to the schools as noted against each in Co1. No.2 with their existing pay and scale of pay plus other admissible allowances per month until further order: They are treated as released from their existing places of posting in the afternoon of 2OlL2 I 2017 and should report for their duty at the places of their transfer on or before 25112/2OL7 and submit joining report to the Head of Office & DDO as indicated against each in Col. No. 3. Their pay and allowances etc will be drawn against the Head of Account under which their pay and allowances etc. are usually drawn. Concerned Heads of Offices are requested to immediately release the employees concerned and send their LPC /Service Book/Personal file etc. to the Heads of Offices as noted in Col. No. 3 against each in due course under intimation to this Directorate. Rr o.ra-.\h (U.K.Chakma) Director of Elementary Education Copy to:- 1. The District Education Officer, Dhalai District for information. 2. The Head of Office & DDO, for information and taking necessary action. 3. Inspector of Schools, for information and taking necessarJi action. -
Ft'c"L:L [ \-/ 1?, I L
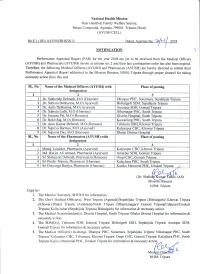
National Health Mission State Health & Family Welfare Society, Pal ace Compound, Agartala-7 9900 1, Tripura (West) (AYUSH CELL) NOTIFICATION \ Performance Appraisal Report (PAR) for the year 2018 are yet to be received from the Medical Officers (AYLJSH) and Pharmacists (AYUSH) shown at column no.2 and their last continuation order has also been expired. Therefore, the following Medical Officers (AYUSH) and Pharmacists (AYUSH) are hereby directed to submit their Performance Appraisal Report addressed to the Mission Director, NHM, Tripura through proper channel for taking necessary action from this end. SL. No Name of the Medical Officers (AYUSH) with Place of posting desisnation ,) I 3 I Dr. Sukhendu Debnath, M.O (Homoeo) Dhanpur PHC, Sonamura, Sepahiiala Tripura 2 Dr. Subrata Debbarrna. M.O (Ayurved) Bishalgarh SDH, Sepahiiala Tripura J Dr. Anita Debbarma, M.O (Ayurved) Amarpur SDH. Gomati Tripura 4 Dr. Subrata Lodh, M.O (Homoeo) Niharnagar PHC, South Tripura 5 Dr. Swapan Pal, M.O (Homoeo) District Hospital, South Tripura 6 Dr. Rohit Rai, M.O (Homoeo) Kowaifung PHC, South Tripura 7 Dr. Arun Kumar Debnath, M.O (Homoeo) Telimura SDH, Khowai Tripura 8 Dr. Supriya Barmal-I, M.O (Ayurved) Kalyanpur CHC, Khowai Tripura 9 Dr. Suparna Das, M.O (Homoeo) Dhalai District Hospital SL. No Name of the Pharmacists (AYUSH) with Place of posting desisnation I 2 3 1 Manoi Talukder, Pharmacist (Ayurved) Kalyanpur CHC, Khowai Tripura 2 Md. Hakim Ali Ahmed, Pharmacist (Ayurved) Amarpur SDH, Gomati Tripura a J Sri Shibaiyoti Debnath, Pharmacist (Homoeo) Ompi CHC, Gomati Tripura 4 Sri Pradip Tripura, Pharmacist (Homoeo) Kalachara PHC, South Tripura 5 Sri Gouranga Baidya, Pharmacist (Homoeo) Kanika Memorial PHC, UnakotiTripura _, b 1nr. -

Brief Industrial Profile of Dhalai District
Government of India Ministry of MSME Brief Industrial Profile of Dhalai District Carried out by MSME-Development Institute Adviser Chowmohani Krishnanagar Road, Agartala-799001,Tripura (Ministry of MSME, Govt. of India,) Phone:0381-2326570,2326576 Fax :0381-2326570 e- mail: [email protected] Web- : www.msmedi-agartala.nic.in Page 1 Contents S. Topic Page No. No. 1. General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 3 1.3 Availability of Minerals. 4 1.4 Forest 6 1.5 Administrative set up 7 2. District at a glance 7 2.1 Existing Status of Industrial Area in the Dhalai District. 11 3. Industrial Scenario Of Dhalai District 11 3.1 Industry at a Glance 11 3.2 Year Wise Trend Of Units Registered 12 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units In The District 13 3.4 Large Scale Industries / Public Sector undertakings 14 3.5 Major Exportable Item 15 3.6 Growth Trend 16 3.7 Vendorisation / Ancillarisation of the Industry 16 3.8 Medium Scale Enterprises 16 3.8.1 List of the units in Dhalai District & near by Area 16 3.8.2 Major Exportable Item 16 3.9 Service Enterprises 16 3.9.1 Potentials areas for service industry 16 3.10 Potential for new MSMEs 17 4. Existing Clusters of Micro & Small Enterprise 18 4.1 Detail Of Major Clusters 18 4.1.1 Manufacturing Sector 18 4.1.2 Service Sector 18 4.2 Details of Identified cluster 18 5. General issues raised by industry association during the course of meeting 18 -19 6. -

Chapter 1 Social Sector
CHAPTER I: SOCIAL SECTOR 1.1 Introduction This Chapter of the Audit Report for the year ended 31 March 2017 deals with the findings on audit of the State Government units under Social Sector. The names of the State Government departments and the break-up of the total budget allocation and expenditure of the State Government under Social Sector during the year 2016-17 are given in Table 1.1.1 . Table: 1.1.1 (` in crore) Total budget Name of the departments Expenditure allocation Education (Higher) Department 194.84 145.29 Education (School) Department 921.11 812.30 Education (Social) Department 427.17 364.43 Elementary Education Department 801.80 669.86 Education (Sports and Youth Programme) Department 139.48 55.98 Food, Civil Supplies and Consumer Affairs Department 122.05 97.94 Family Welfare and Preventive Medicine 412.32 245.37 Health Department 326.77 280.54 Labour Organisation 10.19 8.14 Panchayati Raj Department 234.47 221.41 Public Works (Drinking Water and Sanitation) 289.82 253.17 Department Relief and Rehabilitation Department 30.51 24.66 Rural Development Department 569.89 321.39 Tribal Welfare (Research) Department 3.93 2.73 Kokborok and Other Minority Languages Department 0.38 0.40 Tribal Welfare Department 3,644.45 2,210.17 TRP and PVTG Department 16.86 15.77 Urban Development Department 442.10 390.92 Welfare for SC Department 1,432.03 881.08 Welfare of Minorities Department 111.70 57.25 Welfare of OBC 52.34 30.39 Total number of departments = 21 10,184.21 7,089.19 Source: Appropriation Accounts – 2016-17 Besides the above, the Central Government had transferred a sizeable amount of funds directly to the Implementing Agencies under the Social Sector to different agencies in the State during the year. -
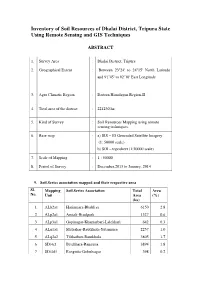
Inventory of Soil Resources of Dhalai District, Tripura State Using Remote Sensing and GIS Techniques
Inventory of Soil Resources of Dhalai District, Tripura State Using Remote Sensing and GIS Techniques ABSTRACT 1. Survey Area : Dhalai District, Tripura 2. Geographical Extent : Between 23o24′ to 24o15′ North Latitude and 91o45′ to 92o10′ East Longitude 3. Agro Climatic Region : Eastern Himalayan Region-II 4. Total area of the district : 221230 ha. 5. Kind of Survey : Soil Resources Mapping using remote sensing techniques. 6. Base map : a) IRS – ID Geocoded Satellite Imagery (1: 50000 scale) b) SOI – toposheet (1:50000 scale) 7. Scale of Mapping : 1 : 50000 8. Period of Survey : December,2013 to January, 2014 9. Soil Series association mapped and their respective area Sl. Mapping Soil Series Association Total Area No. Unit Area (%) (ha) 1 ALb2a1 Harinmara-Bhabliya 6150 2.8 2 ALp2a1 Amtali-Bisalgarh 1327 0.6 3 ALp3a1 Gopinagar-Khamarbari-Lalchhari 642 0.3 4 ALq1a1 Melaghar-Rautkhola-Sutarmura 2257 1.0 5 ALq2a2 Tilthaibari-Rautkhola 3805 1.7 6 SDi4c1 Betchhara-Rangutia 3894 1.8 7 SDi4d1 Rangutia-Gokulnagar 398 0.2 Sl. Mapping Soil Series Association Total Area No. Unit Area (%) (ha) 8 SDn7c(a)1 Kumarghat-Shantipur-Nalifa 1504 0.7 9 SDn7c1 Champamura-Kumarghat 7212 3.3 10 SDn9c(a)1 Shantipur-Kumarghat 730 0.3 11 SDn9c1 Mandirghat-Kumarghat 376 0.2 12 SDn9c2 Kumarghat-Shantipur 2279 1.0 13 SDr4a1 Lembuchhara-Nalifa 88 0.0 14 SDr4b1 Khamting-Betchhara 332 0.2 15 SDr4c1 Nabibari-Bagmara-Rangutia 2301 1.0 16 SDr4c2 Jugalkishor-Rangutia 2013 0.9 17 SDr4d1 Gokulnagar-Rangutia 3075 1.4 18 SDr6c(a)1 Jugalkishor-Taidubari-Khamting 1681 0.8 19 SDr6c1 -

Teacher Education-Joint Review Mission Report on Teacher Education. Tripura. Mission Date
Page 1 of 49 JOINT REVIEW MISSION REPORT ON TEACHER EDUCATION TRIPURA MISSION DATES: JUNE 6 TO13, 2013 Page 2 of 49 Table of contents 1.0 Executive Summary 2.0 Joint Review Mission 2.1 JRM Context 2.1.1 Objectives 2.1.2 Areas of Interest 2.2 Team Members 2.3 Methodology 2.3.1 Preplanning 2.3.2 Visit Schedule 3.0 Teacher Education in Tripura 3.1 Brief History of Tripura and Teacher Education 3.2 Untrained teachers – An early legacy 3.3Present status of teacher education 3.4 Concerns regarding ODL mode 3.5 Recommendations 3.6 State Profile: About the state 3.7 State Profile: Enrolment and Teachers 4.0 Teacher Education Institutions and their role 4.1 State Profile: Teacher education insitutions 4.2 Governance and management of training institutions 4.3 Teacher education institutions--observations 4.3.1 Intake capacities Page 3 of 49 4.4 SCERT 4.4.1 Recommendations 4.5 IASE 4.5.1 Recommendations 4.6 DIETs 4.6.1 Recommendations 5.0 Curriculum and Pedagogy process related to various trainings 5.1 Various Training programmes 5.2 Overall Reflections 5.3 Recommendations 6.0 Conclusions and overall recommendations Page 4 of 49 1.0 Executive Summary 1.1 Key achievements of the states There is a lot of concern and commitment in the government to improve the quality of school education and build linkages with higher education. To strengthen the teacher education in Tripura the state Government has prepared an Annual Work Plan anda Five Year perspective plan for SCERT,IASE, CTE and DIETsconsidering the feasibility and absorption capacity of the state and to enable the DoE ( school) , Government of Tripura, to avail appropriate support from MHRD, GoI. -

Prospectus 2018-2019
PROSPECTUS 2018-2019 GOVT. DEGREE COLLEGE, GANDACHERRA (UGC AFFILIATED) DHALAI, TRIPURA Website: www.gdcgnc.edu.in E-mail: [email protected] Contact: 03826265462/9436471771 COLLEGE PROFILE Govt. Degrre College, Gandacherra(Estt in 2011) is located 117 km away from the capital town of Tripura under Dhalai district. The Panoramic beauty of the college is very charming surrounding by hillocks and the famous water reservoir known as “Dumbur lake”. In spite of being the remotest and geographically most backward place where 95% students are form ST/SC/OBC category and almost all of them are first generation learnres, the college is running with the mission of providing value and quality education and inculcate them among the all section of peoples with the appropriate skills, values and also commitment to create an atmosphere for them so that they can easily compete with the nationally and globally changing atmosphere for livelihood. With this mission the college is adopting various co-curriculum activities like NSS, Swach Bharat Abhiyan and conducting academic & social awarness programmes, workshops, seminers etc. The college has been following the syllabus of Tripura Univerity .The faculties are offered with the academic programme according to university norms and patterns. The present curriculum in the subject like honours in Bengali, History, Education, Political Science with other elective subjects like English, Philosophy, Kokborok, & Soft skill studies and a library to impart the knowledge among the students to compete with the rest of competetive markets in home and abroad. The college has already taken initiative to impart ICT based teaching and learning methods to provide quality education. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Plethora of Plants – Collections of the Botanical Garden, Faculty Of
Nat. Croat. Vol. 24(2), 2015 361 NAT. CROAT. VOL. 24 No 2 361–397* ZAGREB December 31, 2015 professional paper / stručni članak – museal collections / muzejske zbirke DOI: 10.302/NC.2015.24.26 PLETHORA OF PLANTS – ColleCtions of the BotaniCal Garden, faCulty of ScienCe, university of ZaGreB (1): temperate Glasshouse exotiCs – HISTORIC OVERVIEW Sanja Kovačić Botanical Garden, department of Biology, faculty of science, university of Zagreb, marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Kovačić, S.: Plethora of plants – collections of the Botanical garden, Faculty of Science, Univer- sity of Zagreb (1): Temperate glasshouse exotics – historic overview. Nat. Croat., Vol. 24, No. 2, 361–397*, 2015, Zagreb due to the forthcoming obligation to thoroughly catalogue and officially register all living and non-living collections in the european union, an inventory revision of the plant collections in Zagreb Botanical Garden of the faculty of science (university of Zagreb, Croatia) has been initiated. the plant lists of the temperate (warm) greenhouse collections since the construction of the first, exhibition Glasshouse (1891), until today (2015) have been studied. synonymy, nomenclature and origin of plant material have been sorted. lists of species grown (or that presumably lived) in the warm greenhouse conditions during the last 120 years have been constructed to show that throughout that period at least 1000 plant taxa from 380 genera and 90 families inhabited the temperate collections of the Garden. today, that collection holds 320 exotic taxa from 146 genera and 56 families. Key words: Zagreb Botanical Garden, warm greenhouse conditions, historic plant collections, tem- perate glasshouse collection Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno-matematičkog fakulteta Sve- učilišta u Zagrebu (1): Uresnice toplog staklenika – povijesni pregled. -

Biosearch 2004 Report
Biosearch Nyika: Malawi 2004 Edited by Marianne J Overton FOREWORD Peter Overton It is ten years since the Biosearch Nyika project was first mooted and agreement with the Director of National Parks and Wildlife obtained for our exploration of the remoter parts of the Nyika National Park. Over this period the teams have focused mainly on the northern part of the park where patrolling has been very limited and our gathering of intelligence has been most helpful to the Nyika management. In 2004 we undertook the most challenging expedition to date, launched from the extreme north of the park at Uledi, a four-hour drive from Thazima. The team‟s first challenge was to cross the unbridged North Rukuru River with all their supplies. They then had to climb up the western escarpment of the Mpanda ridge to a point on the Mpero River, where they set up a Base Camp, from which to launch out on their surveys. The greatest achievement was to climb both Mpanda and Kawozya and discover the remote Bleak House, now derelict but offering stunning views over Lake Malawi and far beyond. At this point they could certainly claim to be in remote country since this old site is much talked about but very rarely seen by visitors. We have yet to have clear information about who built it, when and why. Perhaps it was a holiday „retreat‟ for Livingstonia or a staging post for missionaries who conducted business on the west of the Nyika National Park and into Zambia. In many ways this expedition was the pinnacle of logistical achievement. -

The Text Is Available (Albeit, Not in the Published Layout) from This Website
The text is available (albeit, not in the published layout) from this website: http://www.fcbs.org/articles/Catalogue_Bromeliaceae_Genera.htm Copyright © 1998 The Marie Selby Botanical Gardens Reprinted with Permission Note: The final printed version of this document can be found in the journal Selbyana 19(1): 91- 121. 1998. This issue is available from the Marie Selby Botanical Gardens for $35, and includes several other Bromeliad-related articles, as well as articles on orchid conservation. Send checks (payable to Selbyana) to Selbyana The Marie Selby Botanical Gardens 811 South Palm Ave. Sarasota FL 34236 USA AN ANNOTATED CATALOGUE OF THE GENERIC NAMES OF THE BROMELIACEAE Jason R. Grant1 University of Alaska Museum, 907 Yukon Drive Fairbanks, Alaska 99775 U.S.A. Gea Zijlstra Department of Plant Ecology and Evolutionary Biology Herbarium, University of Utrecht, Heidelberglaan 2 NL-3584 CS Utrecht, The Netherlands 1 Author for correspondence footnote: ABSTRACT An annotated catalogue of the known generic names of the Bromeliaceae is presented. It accounts for 187 names in six lists: I. Generic names (133), II. Invalid names (7), III. A synonymized checklist of the genera of the Bromeliaceae (56 accepted genera, and 77 synonyms), IV. Nothogenera (bigeneric hybrids) (41), V. Invalid nothogenus (1), and VI. Putative fossil genera (5). Comments on nomenclature or taxonomy are given when necessary to explain problematic issues, and notes on important researchers of the family are intercalated throughout. The etymological derivation of each name is given, including if named after a person, a brief remark on their identity. Appended is a chronological list of monographs of the Bromeliaceae and other works significant to the taxonomy of the family. -

2 the Insect-Pest Situation in Agroforestry
Insect Pests in Agrof orestry Working Paper No. 70 report of a GTZ Fellowship M.P. Singh Rathore Senior Visiting Fellow INTERNATIONAL CENTRE FOR RESEARCH IN AGROFORESTRY Nairobi, Kenya Contents Acknowledgements iv Abstract v 1 Introduction 1 1.1 Sources of information 1 2 The insect-pest situation in agroforestry 3 2.1 Vegetational diversity 4 2.2 Taxonomic alliance 6 2.3 Non-taxonomic alliance 6 2.4 The host range of pests 8 2.5 Biological control potential 8 2.6 Microclimate 10 2.7 Masking effect 11 2.8 Barrier effects 12 2.9 Field configuration and design 12 2.10 Exotic plants and pests 13 2.11 Domestication of plants 15 2.12 Tree-crop competition and nutrition 15 2.13 Management practices 16 3 Strategies for pest management in agroforestry 17 3.1 Choice of species 17 3.2 Microclimate 17 3.3 Field configuration and design 17 3.4 Introduction of barriers .18 3.5 Odoriferous plants 18 3.6 Trap plants 18 3.7 Management practices 18 4 Insects associated with multipurpose trees and shrubs 19 4.1 Literature retrieval 19 4.2 Field observations 19 4.3 Primary sources of information used to compile lists of insects associated with multipurpose trees and shrubs 21 5 Directions for future research 22 6 Conclusion 26 References 27 Appendices 1 Insects associated with multipurpose trees and shrubs—compilation from the literature 35 2 Insects associated with multipurpose trees and shrubs—summary of field observations 67 Acknowledgements The investigations reported in this document were fully funded by the Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ, German Agency for Technical Cooperation) through sponsorship of a Senior Visiting Fellowship, for which the author is grateful.