The Effects of High-Power Microwaves on Comminution and Downstream Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
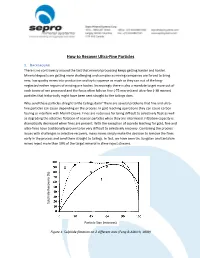
How to Recover Ultra-Fine Particles
How to Recover Ultra-Fine Particles 1. BACKGROUND There is no controversy around the fact that mineral processing keeps getting harder and harder. Mineral deposits are getting more challenging and complex as mining companies are forced to bring new, low quality mines into production and try to squeeze as much as they can out of the long- neglected nether regions of existing ore bodies. Increasingly there is also a mandate to get more out of each tonne of ore processed and the focus often falls on fine (-75 micron) and ultra-fine (-38 micron) particles that historically might have been sent straight to the tailings dam. Why send these particles straight to the tailings dam? There are several problems that fine and ultra- fine particles can cause depending on the process. In gold leaching operations they can cause carbon fouling or interfere with Merrill-Crowe. Fines are notorious for being difficult to selectively float as well as degrading the selective flotation of coarser particles when they are intermixed. Filtration capacity is dramatically decreased when fines are present. With the exception of cyanide leaching for gold, fine and ultra-fines have traditionally proven to be very difficult to selectively recovery. Combining the process issues with challenges in selective recovery, many mines simply make the decision to remove the fines early in the process and send them straight to tailings. In fact, we have seen tin, tungsten and tantalum mines reject more than 10% of the target mineral in slime reject streams. ide Recovery (%) Recovery ide ph l Su Particle Size (microns) Figure 1: Sulphide flotation on 2 different ores (Feng & Aldrich, 1999) 2. -

Gravity Concentration in Artisanal Gold Mining
minerals Review Gravity Concentration in Artisanal Gold Mining Marcello M. Veiga * and Aaron J. Gunson Norman B. Keevil Institute of Mining Engineering, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected] Received: 21 September 2020; Accepted: 13 November 2020; Published: 18 November 2020 Abstract: Worldwide there are over 43 million artisanal miners in virtually all developing countries extracting at least 30 different minerals. Gold, due to its increasing value, is the main mineral extracted by at least half of these miners. The large majority use amalgamation either as the final process to extract gold from gravity concentrates or from the whole ore. This latter method has been causing large losses of mercury to the environment and the most relevant world’s mercury pollution. For years, international agencies and researchers have been promoting gravity concentration methods as a way to eventually avoid the use of mercury or to reduce the mass of material to be amalgamated. This article reviews typical gravity concentration methods used by artisanal miners in developing countries, based on numerous field trips of the authors to more than 35 countries where artisanal gold mining is common. Keywords: artisanal mining; gold; gravity concentration 1. Introduction Worldwide, there are more than 43 million micro, small, medium, and large artisanal miners extracting at least 30 different minerals in rural regions of developing countries (IGF, 2017) [1]. Approximately 20 million people in more than 70 countries are directly involved in artisanal gold mining (AGM), with an estimated gold production between 380 and 450 tonnes per annum (tpa) (Seccatore et al., 2014 [2], Thomas et al., 2019 [3], Stocklin-Weinberg et al., 2019 [4], UNEP, 2020 [5]). -

Application of Falcon Centrifuge As a Cleaner Alternative for Complex Tungsten Ore Processing
minerals Article Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing Yann. Foucaud 1,* , Quentin Dehaine 1,2 , Lev. O. Filippov 1,3,* and Inna V. Filippova 1,3 1 GeoRessources Laboratory CNRS, Université de Lorraine, F54000 Nancy, France 2 Camborne School of Mines, University of Exeter, Penryn Campus, Cornwall TR10 9EF, UK 3 National University of Science and Technology MISIS, 119049 Moscow, Russia * Correspondence: [email protected] (Y.F.); lev.fi[email protected] (L.O.F.) Received: 15 June 2019; Accepted: 17 July 2019; Published: 19 July 2019 Abstract: Scheelite (CaWO4) is one of the main raw material for the production of tungsten. It is usually encountered in skarn deposits where it is commonly associated with other calcium minerals as fluorite, apatite, and calcium silicates. Worldwide, scheelite is upgraded to the chemical grades by direct flotation, but the separation efficiency remains limited due to similar flotation behaviors of scheelite and gangue minerals with fatty acid. The only solutions used to overcome this issue involve high energy consumption or ecotoxic reagents. In the present study, a novel method based on the use of a centrifugal Falcon concentrator was investigated to perform an efficient elimination of gangue minerals and fine particles as well as an acceptable scheelite recovery enabling a decrease of the flotation reagents consumption. The performances of the two types of laboratory Falcon bowls, Falcon UltraFine (UF) and Falcon Semi-Batch (SB), were modeled using the design of experiments (DoE) methodology, which allowed to determine the best operating parameters for each bowl. -

GOLD EXTRACTION As the Discovery Rate and Grades Fall, John Chadwick
GOLD EXTRACTION Midas mission As the discovery rate and grades fall, John Chadwick looks at ideas and technologies to get the most out of the world’s gold deposits he declining rate of new gold discoveries North America, although with significantly less MDM Engineering Group has signed an EPC contract for the execution of a 2.4 Mt/y gold and grades across the global market new ounces of 290 Moz, and with a much lower plant for African Barrick Gold’s (ABG) Tduring the last decade has accelerated grade of 1.3 g/t. Bulyanhulu Process Plant Expansion project in over the last four years. Data and analysis from Europe had the third most new discoveries Tanzania. ABG is the largest gold producer in Tanzania with a portfolio of four operating IntierraRMG reveals that the two-year period with 240 Moz but with a higher grade than North mines and is the owner and operator of from 2003 to 2004 was the best in the study America of 2.0 g/t. South America recorded 188 Bulyanhulu Gold Mine. MDM successfully range, with over 400 Moz of new gold Moz, whilst Australasia saw 74 Moz of new completed the bankable feasibility study in December 2011 followed by value engineering discovered. This includes Inferred, Indicated and discoveries with an average grade of 1.4 g/t. in March and April 2012. MDM is now executing Measured ounces with an average grade of 1.65 Glen Jones, Western Hemisphere Director for the project which includes construction of a g/t. In contrast, 2005 and 2006 had the lowest IntierraRMG concluded, “With global drilling new 2.4 Mt/y CIL (carbon in leach) facility to process tailings reclaimed from the historical number, with just over 150 Moz of new gold activity waning, IntierraRMG forecasts that the tailings concurrently with current flotation discovered - albeit with a similar grade. -

Dense Media Separation – a Valuable Process for Preconcentration
1 Dense Media Separation – a Valuable Process for Preconcentration M Lundt1, I Grewal 2 1. Senior Process Engineer, Sepro Mineral Systems, 101A – 9850 – 201 Street, Langley, British Columbia, Canada V1M 4A3, [email protected] 2. President, Met-Solve Laboratories Inc. 101B – 9850 – 201 Street, Langley, British Columbia, Canada V1M 4A3, [email protected] ABSTRACT Dense medium separation (DMS) is one of several preconcentration methods used for early waste rejection from run-of-mine ores at relatively coarse particle sizes prior to additional milling and beneficiation. DMS has been used extensively in the coal, diamond, mineral sands, base metals and iron ore industries to produce either a final product or as a preconcentration step. Laboratory scale and pilot test work at the Met-Solve laboratory in Canada has demonstrated successful separation and upgrading of other minerals such as lead, zinc, copper and lithium based ores using DMS. This paper reviews historical dense media systems, results from the pilot-scale multi-stage DMS Separator “Condor” system, as well as production plants and associated laboratory heavy liquid separation (HLS) test work on various minerals. The mineral systems tested included a lithium bearing ore and a fluorspar ore. Other minerals were tested but are not reported in this paper. Pilot plant test work on a fluorspar ore showed that 41.6% of the mass could be rejected to the tailings stream while 95.3% of the CaF2 was recoverable to the product stream. On a spodumene sample that was tested, it was possible to reject 55% of the mass to tailings while recovering over 90% of the lithium. -

The Potential for Dense Medium Separation of Mineral Fines Using a Laboratory Falcon Concentrator
Marion et al. The Potential for Dense Medium Separation of Mineral Fines Using a Laboratory Falcon Concentrator The Potential for Dense Medium Separation of Mineral Fines Using a Laboratory Falcon Concentrator C. Marion1, H. Williams1, R. Langlois1, O. Kökkılıç1, F. Coelho2, M. Awais3, N.A. Rowson2, K.E. Waters1* 1: Department of Mining and Materials Engineering, McGill University, 3610 University, Montreal, Quebec H3A 0C5, Canada 2: School of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom 3: School of Metallurgy and Materials Science, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom *Author for Correspondence: [email protected] Abstract Dense medium separation (DMS) is a technique used to separate particles based on specific gravity. Conventional DMS is, however, limited to coarse particle sizes and is not practical when processing fines. To improve the separation efficiency when processing fine particles, centrifugal separators have been employed. This work investigated DMS in a lab centrifuge and a modified Falcon Concentrator, in order to process the slimes of a rare earth ore. It has been shown that centrifugal concentration using a dense medium is possible when recovering values from a slimes fraction. Both the lab centrifuge and modified Falcon Concentrator resulted in a similar performance. Keywords: Dense medium separation; Centrifugal separation; Falcon Concentrator; Fine particle separations 1 Marion et al. The Potential for Dense Medium Separation of Mineral Fines Using a Laboratory Falcon Concentrator 1 Introduction Dense medium separation (DMS) is a process by which particles are separated based on differences in specific gravity (SG). The process can be controlled with a high degree of precision over a wide range of separating densities and is commonly applied as a pre- concentration step for minerals (such as cassiterite) and in the separation of coal from contaminants (Wills and Finch, 2016). -

2014 Preliminary Economic Assessment of the Eagle Mountain
PRELIMINARY ECONOMIC ASSESSMENT OF THE EAGLE MOUNTAIN SAPROLITE GOLD PROJECT, GUYANA for Report No. 977 A.C.A. Howe International Limited Toronto, Ontario, Canada Wm. Douglas Roy, M.A.Sc., P.Eng. Ian D. Trinder, M.Sc., P.Geo. Alex Lum, P.Eng. Mauritz Lundt, B.Eng., P.Eng. Effective Date: June 15, 2014 Signature Date: September 12, 2014 Effective Date: June 15, 2014 Signature Date: September 12, 2014 Report Number: 977 Client Reference: GOLDSOURCE MINES INC. PRELIMINARY ECONOMIC ASSESSMENT OF THE EAGLE MOUNTAIN SAPROLITE GOLD PROJECT, GUYANA Authors: Wm. Douglas Roy, M.A.Sc., P.Eng. “Signed and Sealed” Associate Mining Engineer _____________________ ACA Howe International Ian D. Trinder, M.Sc., P.Geo. “Signed and Sealed” Senior Geologist _____________________ ACA Howe International Alex Lum, P.Eng. “Signed and Sealed” Senior Metallurgist _____________________ Met-Solve Laboratories Inc. Mauritz Lundt, B.Eng., P.Eng. “Signed and Sealed” Senior Process Engineer _____________________ Sepro Mineral Systems 365 Bay Street, Suite 501, Toronto, ON, Canada, M5H2V1 (t) +1.416.368.7041, (f) +1.416.368.2579 Wingbury Courtyard Business Village, Upper Wingbury Farm, Wingrave, Aylesbury, Buckinghamshire, HP22 4LW, U.K. (t) +44.1442.873.398 website: www.acahowe.ca; www.acahowe.co.uk Office Locations: Toronto – London – Halifax TABLE OF CONTENTS 1 SUMMARY ......................................................................................................................................... 1 1.1 General ............................................................................................................................ -

Convention Program & Expo Guide
THE CHANGING FACE OF MINING CONVENTION PROGRAM & EXPO GUIDE PROGRAMME DU CONGRÈS et GUIDE DE L’EXPO LE VISAGE CHANGEANT DE L’INDUSTRIE MINIÈRE ATION • IMPACT • INFORMATION • INNOVATION • INTELLIGENCE • INFLUENCE • APRIL 28–MAY 1, 2019 | 28 AVRIL AU 1ER MAI 2019 PALAIS DES CONGRÈS DE MONTRÉAL | MONTRÉAL, QUÉBEC | CONVENTION.CIM.ORG TECHNICAL PROGRAM MONDAY PROGRAMME TECHNIQUE LUNDI AM (10:50) Innovation, Automation and Optimizing Value 513A Data Managing Innovation 513C Operations: Case Studies and Data Driven Optimization in Mining 514B Best Practices Mining in Perspective and Looking Ahead 515A People and Communities Social License: From 101 to Complex Strategies 516A Sustainable Mining Holistic Mine Water Management 513E From Rocks to Resources Mineral Property Valuation Methods 516D Health and Safety Education and Risk Management 516B 7th North American Iron Ore Iron Ore Resources 516C Symposium PM (14:00) Innovation, Automation and Autonomous Mining 513A Data AI and Mining 513C Operations: Case Studies and Rock Engineering I (Underground) 514B Best Practices Processing 515A People and Communities Canadian Mining Education Forum: Integrating 516A Social Responsibility Sustainable Mining A Fresh Take on Maximizing Value 513E From Rocks to Resources Risk Management for Practitioners 516D Health and Safety J.T. Ryan Award Winners 2017 516B 7th North American Iron Ore Projects Overview 516C Symposium CONVENTION.CIM.ORG | 43 CIM CONVENTION | 2019 | CONGRÈS DE L’ICM MONDAY, APRIL 29 | 10:50 - 12:30 ROOM: 513A OPTIMIZING VALUE INNOVATION, -

49Th ANNUAL CANADIAN MINERAL PROCESSORS CONFERENCE
Comminution Circuit Design Considerations 13:00 A New Method for the Stabilization of CANADIAN MINERAL PROCESSORS Paul Staples Arsenic from Enargite Concentrates Improving Concentrate Grade through Smart Design and Piloting Gabriel Garcia Curiel 14:25 Virginia Lawson Use of Functional Performance Models to 10:40 Increase Plant Grinding Efficiency 13:25 Economic Evaluation of Activated Carbon for Rob McIvor Gold Milling Circuits Online Elemental Analysis of Slurry using Mark Somppi Break Laser Induced Breakdown Spectroscopy Lauri Koresaar 11:05 15:20 Mini- 13:50 Selecting the Correct Cyanide Destruction Technical Review and Evaluation of Ore Process for Your Operation Sorting Technology Incorporating Results The Removal of Iron-bearing Silicate Schedule Minerals from a Hard Rock Lithium Ore Anca Nacu from Four Properties Brent Hilscher Charlotte Gibson TUESDAY Lunch 11:30 Break 15:45 GOLD 8:30 Spiral Surface Property Investigation FLOTATION 13:00 OPENING REMARKS Wynand Erasmus 14:45 Selecting the number of increments for Scott Martin 16:10 Improvement of Valuable Mineral Transport daily metallurgical samples: Application to PLENARY PRESENTATION and Gangue Drainage in Froth Flotation Goldcorp Eleonore Gold Ore Concentrator, Flash Flotation Circuit Design Considerations Quebec, Canada 15:10 8:45 Ben Murphy 13:25 Linking Flotation Efficiency to Bubble Size The Need to Innovate: Celebrate the Past.... Day 1 Concludes 16:35 Jose R. Hernandez-Aguilar Assessing the Total Cost of Representativity Look to the Future in Flotation Plants Dominic -

Cutting Waste the Latest in Gravity Techniques, Ore Sorting and Magnetic Separation to Raise Separation Efficiency and Reduce Concentration Costs
PRECONCENTRATION John Chadwick examines Cutting waste the latest in gravity techniques, ore sorting and magnetic separation to raise separation efficiency and reduce concentration costs “Sensor based automatic ore sorting technology. In bulk tests a very high waste The huge Natalka gold project, owned by Polyus, includes a three-stage gravity circuit; by technologies are used to efficiently remove reject rate of 92.2% was achieved with no gold far the world’s largest – with no less than 52 waste and sub marginal grade material from detected by assay in the waste (98.1% recovery centrifugal gravity concentrators, with sizes up crushed and screened run-of-mine (ROM) ore, assumed based on assay detection limit). As to 42 in. There are multiple concentrators from the two world leaders - FLSmidth’s Knelson producing a coarse (typically 10 mm) upgraded nearly 100% of the gold was recovered, a high > brand and Sepro Mineral Systems’ Falcon preconcentrate at an early stage in the mineral degree of liberation of mineralised quartz reef brand processing circuit. This avoids additional energy from gangue presumably exists at particle sizes intensive downstream processing of this between 25 mm and 60 mm. rejected component of the ROM feed, leading to “The financial modelling confirms robust doubled with ore sorting, from a bulk ROM lower unit operating costs and reduced tailings economics for the project. The base case grade of 0.8 g/t to a mill feed grade of 1.93 g/t disposal volumes.” These are comments from modelling of this potential operation indicates The flow sheet assumes 30% of the crushed and Tony Parry and G van Wyk of Steinert Australia that the grade of plant feed can be more than screened stockpile tonnage prior to sorting is - in their paper Upgrading Low-grade Gold Ore Stockpiles by Preconcentration Using Ore Sorting – an Assessment of the Economic Impact and Viability, presented at the 13th AusIMM Mill Operators’ Conference, October 2016. -

Spreading the WORD
CANADA ’ S FIRST MINING PUBLICATION CACANADIANNADIAN Mining JournalOctober 2014 Mwww.canadianminingjournal.comining Journal Spreading THE WORD Goldcorp’s Éléonore Mine in Quebec is ‘the place’ to work MINE RUNNER 35 ELGIN STREET, NORTH THORNBURY, ONTARIO 1.519.599.2015 www.rockbreaker.com CANADA, N0H 2P0 Departments 5 Editorial CANADIANCANADIAN This month Editor Russell Noble takes a whimsical look at what it’s like to be Mining Journal driven to near insanity by the swarms of insects that crews must endure while working at remote sites. CONTENTS 6 Investing Columnist Ned Goodman talks about money and the job situation. MINING IN QUEBEC 9 Law Goldcorp goes to great lengths Norton Rose Fulbright Canada’s Crae to help ensure that its Éléonore Garrett and Jean-Philipe Buteau explain Mine in northern Quebec meets Quebec’s Plan Nord program in detail. 10 high standards both in terms of production and accommodations. 44 Leadership Correspondent Carl Friesen discusses why companies need to offer more than mining skills when working on projects in foreign countries. 48 Company Profile Yokohama Tire (Canada) Inc is the sub- ject of this month’s Company Profile. 52 In My Mine(d) Doug Morrison, President of the Centre Stornoway’s Renard Mine for Excellence in Mining Innovations 38 continues on its way to (CEMI) of Sudbury talks about the orga- becoming Quebec’s first nization and its mandate to develop a Diamond mine. comprehensive, multi-disciplinary approach to making mining a safer and environmentally friendly industry. IAMGOLD transitions its 16 Westwood project from 54 Unearthing Trends a development site into a A regular column by Ernst & Young LLP, working mine with an Vancouver. -

My Experiences with Falcon Continuous Concentrators
October 2010 MORE THAN A DECADE OF EXPERIENCE WITH CONTINUOUS FALCON CONCENTRATORS AT THE TANTALUM MINING CORPORATION OF CANADA’S LAC DU BONNET OPERATION by Claude Deveau, P. Eng. [email protected] Chief Metallurgist Continuous Falcon Concentrator October 2010 Page 1 of 17 Table of Contents Page Background……………………………………………………………………… 3 About the Author………………………………………………………………. 4 Author’s Overview………………………………………………………………. 4 History of Continuous Falcon at Tanco………………………………………… 5 Metallurgical Performance of the Continuous Falcon…………………….…. 7 Mechanical Integrity of the Continuous Falcon………………………………. 9 Wear Parts………………………………………………………………………. 10 Mechanical Components……………………………………………………….. 14 History of Ultra-Fine (UF) Falcon Concentrator at Tanco.…………………. 15 Performance of the UF Falcon Concentrator Treating Very Fine Tantalum. 16 Mechanical Integrity of the UF Falcon Concentrator………………………… 16 Conclusion………………………………………………………………………. 17 October 2010 Page 2 of 17 Background Tantalum minerals break down into extremely fine particles when undergoing size reduction via crushing and grinding. After grinding, gravity concentration is typically used to recover Tantalum. Conventional gravity concentration technology operates at 1 G or less and had been found to be inefficient for recovery of tantalum fines at Tanco. Tanco conducted an evaluation of its ‘sand’ circuit (Particle sizes in the range of 300µm x 20µm) in 1994. The results showed that there were losses due to the limitations of the conventional gravity separation equipment that consisted of a combination of Holman sand tables and spirals. Even though four separate particle size classes were created and treated separately, losses were substantial. These losses were characterized in order to evaluate whether any of the new (at that time) enhanced gravity concentration technologies could be used to scavenge Tanco’s sand circuit tailings thereby improving overall plant recovery.