Bandgap Opening in Hydrogenated Germanene Q
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silicene/Germanene on Mgx2 (X = Cl, Br, and I) for Li-Ion Battery Applications
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CURVE/open Silicene/germanene on MgX2 (X = Cl, Br, and I) for Li-ion battery applications Zhu, J, Chroneos, A & Schwingenschlögl, U Author post-print (accepted) deposited by Coventry University’s Repository Original citation & hyperlink: Zhu, J, Chroneos, A & Schwingenschlögl, U 2016, 'Silicene/germanene on MgX2 (X = Cl, Br, and I) for Li-ion battery applications' Nanoscale, vol 8, pp. 7272-7277 https://dx.doi.org/10.1039/C6NR00913A DOI 10.1039/C6NR00913A ISSN 2040-3364 ESSN 2040-3372 Publisher: Royal Society of Chemistry Copyright © and Moral Rights are retained by the author(s) and/ or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This item cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder(s). The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. This document is the author’s post-print version, incorporating any revisions agreed during the peer-review process. Some differences between the published version and this version may remain and you are advised to consult the published version if you wish to cite from it. Silicene/Germanene on MgX2 (X = Cl, Br, and I) for Li-Ion Battery Applications Jiajie Zhu1, Alexander Chroneos2;3;a and Udo Schwingenschl¨ogl1;b 1PSE Division, KAUST, Thuwal 23955-6900, Kingdom of Saudi Arabia 2Department of Materials, Imperial College, London SW7 2AZ, United Kingdom 3Faculty of Engineering and Computing, Coventry University, Priory Street, Coventry CV1 5FB, United Kingdom aEmail: [email protected] bEmail: [email protected] September 30, 2015 Abstract Silicene is a promising electrode material for Li-ion batteries due to a high Li capacity and low Li diffusion barrier. -

Application of Silicene, Germanene and Stanene for Na Or Li Ion Storage: a Theoretical Investigation
Application of silicene, germanene and stanene for Na or Li ion storage: A theoretical investigation Bohayra Mortazavi*,1, Arezoo Dianat2, Gianaurelio Cuniberti2, Timon Rabczuk1,# 1Institute of Structural Mechanics, Bauhaus-Universität Weimar, Marienstr. 15, D-99423 Weimar, Germany. 2Institute for Materials Science and Max Bergman Center of Biomaterials, TU Dresden, 01062 Dresden, Germany Abstract Silicene, germanene and stanene likely to graphene are atomic thick material with interesting properties. We employed first-principles density functional theory (DFT) calculations to investigate and compare the interaction of Na or Li ions on these films. We first identified the most stable binding sites and their corresponding binding energies for a single Na or Li adatom on the considered membranes. Then we gradually increased the ions concentration until the full saturation of the surfaces is achieved. Our Bader charge analysis confirmed complete charge transfer between Li or Na ions with the studied 2D sheets. We then utilized nudged elastic band method to analyze and compare the energy barriers for Li or Na ions diffusions along the surface and through the films thicknesses. Our investigation findings can be useful for the potential application of silicene, germanene and stanene for Na or Li ion batteries. Keywords: Silicene; germanene; stanene; first-principles; Li ions; *Corresponding author (Bohayra Mortazavi): [email protected] Tel: +49 157 8037 8770, Fax: +49 364 358 4511 #[email protected] 1. Introduction The interest toward two-dimensional (2D) materials was raised by the great success of graphene [1–3]. Graphene is a zero-gap semiconductor that present outstanding mechanical [4] and heat conduction [5] properties, surpassing all known materials. -

Cooperative Electron-Phonon Coupling and Buckled Structure in Germanene on Au(111) Jincheng Zhuang University of Wollongong, [email protected]
University of Wollongong Research Online Australian Institute for Innovative Materials - Papers Australian Institute for Innovative Materials 2017 Cooperative Electron-Phonon Coupling and Buckled Structure in Germanene on Au(111) Jincheng Zhuang University of Wollongong, [email protected] Nan Guo Dalian University of Technology Zhi Li University of Wollongong, [email protected] Xun Xu University of Wollongong, [email protected] Jiaou Wang Chinese Academy of Sciences See next page for additional authors Publication Details Zhuang, J., Guo, N., Li, Z., Xu, X., Wang, J., Zhao, J., Dou, S. Xue. & Du, Y. (2017). Cooperative Electron-Phonon Coupling and Buckled Structure in Germanene on Au(111). ACS Nano, 11 (4), 3553-3559. Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Cooperative Electron-Phonon Coupling and Buckled Structure in Germanene on Au(111) Abstract Germanene, a single-atom-thick germanium nanosheet in a honeycomb lattice, was proposed to be a Dirac fermion material beyond graphene. We performed scanning tunneling microscopy and in situ Raman spectroscopy studies combined with first-principles calculations on the atomic structures and the electronic and phonon properties of germanene on Au(111). The low-buckled 1 x 1 germanene honeycomb lattice was determined to exist in an unexpected rectangular √7 x √7 superstructure. Through in situ Raman measurements, distinctive vibrational phonon modes were discovered in √7 x √7 germanene, revealing the special coupling between the Dirac fermion and lattice vibrations, namely, electron-phonon coupling (EPC). The significant enhancement of EPC is correlated with the tensile strain, which is evoked by the singular buckled structure of √7 x √7 germanene on the Au(111) substrate. -

Exotic Forms of Low-Dimensional Epitaxial Silicon and Xenes
Exoc forms of low-dimensional epitaxial silicon and Xenes Guy Le Lay, Eric Salomon and Thierry Angot Aix-Marseille University, CNRS-PIIM, France Workshop on Chiral Modes in Opcs and Electronics of 2D Systems, Aussois, France, Nov. 26-28, 2018 D Epitaxial silicene archetype Kagome silicene Large area stanene structure on Ag(111)4x4 on Al(111)3x3 (3x3 nm2) on Ag(111)√3x√3 Co-workers Europe T. Angot and E. Salomon, Marseille, France Y. Sassa and coll., Uppsala, Sweden H. Sahin and F. Iyikanat, Izmir, Turkey Japan J. Yuhara and coll., Nagoya, Japan The Hunt for the Topological Qubit When a TI is coated by an s-wave superconductor (SC), the superconducng vorces are Majorana fermions—they are their own anAparAcles. Exchanging or braiding Majorana vorces, as sketched here, leads to non-abelian stasAcs. Such behavior could form the basis piece of hardware (Majorana Qubit) for topological quantum compung. Xiao-Liang Qi and Shou-Cheng Zhang, Physics Today Jan. 2010, 33 The Challenge: the Hardware QSHE « Experimental synthesis and characterizaon of 2D Topological Insulators remain a major challenge at present, offering outstanding opportuniAes for innovaon and breakthrough. » Kou et al., J. Phys. Chem. Le. 2017, 8, 1905 The way: Nanoarchitectonics, i.e., create atomically controlled ar9ficial structure by design The ar9ficial Xenes What about Si, Ge, Sn, and Pb group 14 arficial counterparts of graphene? XXXXXXXXXXXXXXXXXXXXX Graphene Flat No band gap Minute SOC Silicene Germanene Increasing Spin Orbit Coupling Stanene 82 Plumbene Pb The hardware beyond graphene First predic9on in 1994, 10 years before the isola9on of graphene! “Theorecal Possibility of Stage Corrugaon in Si and Ge Analogs of graphite” ⇒ K. -

Recent Advances in Synthesis, Properties, and Applications of Phosphorene
www.nature.com/npj2dmaterials REVIEW ARTICLE OPEN Recent advances in synthesis, properties, and applications of phosphorene Meysam Akhtar1,2, George Anderson1, Rong Zhao1, Adel Alruqi1, Joanna E. Mroczkowska3, Gamini Sumanasekera1,2 and Jacek B. Jasinski2 Since its first fabrication by exfoliation in 2014, phosphorene has been the focus of rapidly expanding research activities. The number of phosphorene publications has been increasing at a rate exceeding that of other two-dimensional materials. This tremendous level of excitement arises from the unique properties of phosphorene, including its puckered layer structure. With its widely tunable band gap, strong in-plane anisotropy, and high carrier mobility, phosphorene is at the center of numerous fundamental studies and applications spanning from electronic, optoelectronic, and spintronic devices to sensors, actuators, and thermoelectrics to energy conversion, and storage devices. Here, we review the most significant recent studies in the field of phosphorene research and technology. Our focus is on the synthesis and layer number determination, anisotropic properties, tuning of the band gap and related properties, strain engineering, and applications in electronics, thermoelectrics, and energy storage. The current needs and likely future research directions for phosphorene are also discussed. npj 2D Materials and Applications (2017) 1:5 ; doi:10.1038/s41699-017-0007-5 INTRODUCTION properties, including high carrier mobility, ultrahigh surface area, Since the discovery of graphene in 2004 (ref. 1), there has been a excellent thermal conductivity, and quantum confinement effect 9 quest for new two-dimensional (2D) materials aimed at fully have been well documented. However, the lack of band gap is a exploring new fundamental phenomena stemming from quantum serious limitation for the use of graphene in electronic devices. -

Resonance Raman Spectroscopy of Silicene and Germanene
Resonance Raman spectroscopy of silicene and germanene Gergő Kukucska,y Viktor Zólyomi,z and János Koltai∗,y yDepartment of Biological Physics, Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary zSchool of Physics and Astronomy, University of Manchester, Oxford Road, Manchester M13 9PL, UK E-mail: [email protected] Abstract We model Raman processes in silicene and germanene involving scattering of quasi- particles by, either, two phonons, or, one phonon and one point defect. We compute the resonance Raman intensities and lifetimes for laser excitations between 1 and 3 eV using a newly developed third-nearest neighbour tight-binding model parametrized from first principles density functional theory. We identify features in the Raman spectra that are unique to the studied materials or the defects therein. We find that in silicene, a new Raman resonance arises from the 2:77 eV π − σ plasmon at the M point, measurably higher than the Raman resonance originating from the 2:12 eV π plasmon energy. We show that in germanene, the lifetimes of charge carriers, and thereby the linewidths of arXiv:1808.01354v3 [cond-mat.mtrl-sci] 3 Jan 2019 the Raman peaks, are influenced by spin-orbit splittings within the electronic structure. We use our model to predict scattering cross sections for defect induced Raman scat- tering involving adatoms, substitutional impurities, Stone-Wales pairs, and vacancies, and argue that the presence of each of these defects in silicene and germanene can be qualitatively matched to specific features in the Raman response. 1 Keywords Raman scattering, silicene, germanene, Density Functional Theory, Tight Binding, Spin-orbit coupling Introduction Graphene-like hexagonal materials composed of silicon or germanium are unique two-dimensional (2D) crystals with a promising future in nanoelectronics. -
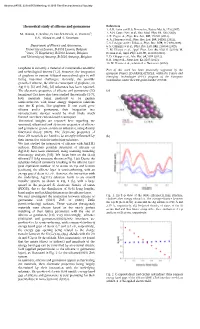
Theoretical Study of Silicene and Germanene References 1
Abstract #755, 223rd ECS Meeting, © 2013 The Electrochemical Society Theoretical study of silicene and germanene References 1. A.K. Geim and K.S. Novoselov, Nature Mat. 6, 183 (2007). M. Houssa, E. Scalise, B. van den Broek, G. Pourtois*), 2. A.H. Castro Neto et al., Rev. Mod. Phys. 81, 109 (2009). 3. P. Vogt et al., Phys. Rev. Lett. 108, 155501 (2012). V.V. Afanas'ev, and A. Stesmans 4. A. Fleurence et al., Phys. Rev. Lett. 108, 245501 (2012). 5. S. Lebègue and O. Eriksson, Phys. Rev. B 79, 115409 (2009). Department of Physics and Astronomy, 6. S. Cahangirov et al., Phys. Rev. Lett. 102, 236804 (2009). University of Leuven, B-3001 Leuven, Belgium 7. M. Houssa et al., Appl. Phys. Lett. 96, 082111 (2010); M. *) imec, 75 Kapeldreef, B-3001 Leuven, Belgium Houssa et al., Appl. Phys. Lett. 97, 112106 (2010). and University of Antwerp, B-2610 Antwerp, Belgium 8. D. Chiappe et al., Adv. Mat. 24, 5088 (2012). 9. B. Feng et al., Nano Lett. 12, 3507 (2012). 10. M. Houssa et al., submitted to Nanoscale (2012). Graphene is currently a material of considerable scientific Part of this work has been financially supported by the and technological interest [1,2]. However, the integration European Project 2D-NANOLATTICES, within the Future and of graphene in current Si-based nanotechnologies is still Emerging Technologies (FET) program of the European facing important challenges. Recently, the possible Commission, under the FET-grant number 270749. growth of silicene, the silicon counterpart of graphene, on Ag(111) [3] and ZrB2 [4] substrates has been reported. -

New Phases of Germanene
New Phases of Germanene V. Ongun Oz¸celik,¨ 1, 2 E. Durgun,1, 2 and S. Ciraci3 1UNAM-National Nanotechnology Research Center, Bilkent University, 06800 Ankara, Turkey 2Institute of Materials Science and Nanotechnology, Bilkent University, Ankara 06800, Turkey 3Department of Physics, Bilkent University, Ankara 06800, Turkey Germanene, a graphene like single layer structure of Ge, has been shown to be stable and recently grown on Pt and Au substrates. We show that a Ge adatom adsorbed to germanene pushes down the host Ge atom underneath and forms a dumbbell structure. This exothermic process occurs spon- taneously. The attractive dumbbell-dumbbell interaction favors high coverage of dumbbells. This letter heralds stable new phases of germanene, which are constructed from periodically repeating coverage of dumbbell structures and display diversity of electronic and magnetic properties. Three dimensional (3D) layered bulk phases, such as graphite,[1] BN[2] and MoS2[3] have led to the synthesis of single layer, honeycomb structures of those materi- als, which were initially conjectured to be unstable.[4{ 7] The existence of single layer, graphene like struc- tures of other Group IV elements, like Si and Ge, have been ruled out since these elements do not have lay- ered allotropes like graphite that would allow the syn- thesis of their single layer structures. Surprisingly, based on state of the art first-principles calculations, silicene,[8, 9] germanene,[10, 11] most of III-V and II-VI compounds[9, 11] and several transition metal dichalco- genides and oxides[12] have been shown to form stable, single layer honeycomb structures. Moreover, it has been also shown that silicene and germanene share several of FIG. -

Structural and Electronic Properties of Germanene on Mos2
week ending PRL 116, 256804 (2016) PHYSICAL REVIEW LETTERS 24 JUNE 2016 Structural and Electronic Properties of Germanene on MoS2 L. Zhang,1 P. Bampoulis,1 A. N. Rudenko,2 Q. Yao,1 A. van Houselt,1 B. Poelsema,1 M. I. Katsnelson,2 and H. J. W. Zandvliet1,* 1Physics of Interfaces and Nanomaterials, MESA+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands 2Institute for Molecules and Materials, Radboud University, Heijendaalseweg 135, 6525 AJ Nijmegen, The Netherlands (Received 24 January 2016; published 21 June 2016) To date germanene has only been synthesized on metallic substrates. A metallic substrate is usually detrimental for the two-dimensional Dirac nature of germanene because the important electronic states near the Fermi level of germanene can hybridize with the electronic states of the metallic substrate. Here we report the successful synthesis of germanene on molybdenum disulfide (MoS2), a band gap material. Preexisting defects in the MoS2 surface act as preferential nucleation sites for the germanene islands. The lattice constant of the germanene layer (3.8 Æ 0.2 Å) is about 20% larger than the lattice constant of the MoS2 substrate (3.16 Å). Scanning tunneling spectroscopy measurements and density functional theory calculations reveal that there are, besides the linearly dispersing bands at the K points, two parabolic bands that cross the Fermi level at the Γ point. DOI: 10.1103/PhysRevLett.116.256804 The discovery that graphene, a single layer of sp2 edges of the material [6,7]. The two topologically protected hybridized carbon atoms arranged in a honeycomb registry, spin-polarized edge modes have opposite propagation is stable, has resulted in numerous intriguing and exciting directions and therefore the charge conductance vanishes, scientific breakthroughs [1,2]. -

Spin Structure Factors of Doped Monolayer Germanene in the Presence of Spin-Orbit Coupling
www.nature.com/scientificreports OPEN Spin structure factors of doped monolayer Germanene in the presence of spin‑orbit coupling Farshad Azizi & Hamed Rezania* In this paper, we present a Kane‑Mele model in the presence of magnetic feld and next nearest neighbors hopping amplitudes for investigations of the spin susceptibilities of Germanene layer. Green’s function approach has been implemented to fnd the behavior of dynamical spin susceptibilities of Germanene layer within linear response theoryand in the presence of magnetic feld and spin‑orbit coupling at fnite temperature. Our results show the magnetic excitation mode for both longitudinal and transverse components of spin tends to higher frequencies with spin‑orbit coupling strength. Moreover the frequency positions of sharp peaks in longitudinal dynamical spin susceptibility are not afected by variation of magnetic feld while the peaks in transverse dynamical susceptibility moves to lower frequencies with magnetic feld. The efects of electron doping on frequency behaviors of spin susceptibilities have been addressed in details. Finally the temperature dependence of static spin structure factors due to the efects of spin‑orbit coupling, magnetic feld and chemical potential has been studied. A lot of theoretical and experimental studies have been performed on Graphene as a one-atom-thick layer of graphite since it’s fabrication1. Te low energy linear dispersion and chiral property of carbon structure leads to map the nearest neighbor hopping tight binding hamiltonian which at low energy to a relativistic Dirac Hamiltonian for massless fermions with Fermi velocity vF . Novel electronic properties have been exhibited by Graphene layer with a zero band gap which compared to materials with a non-zero energy gap. -

Tuning the Analog and Digital Performance of Germanene
Materials Science in Semiconductor Processing 80 (2018) 18–23 Contents lists available at ScienceDirect Materials Science in Semiconductor Processing journal homepage: www.elsevier.com/locate/mssp Tuning the analog and digital performance of Germanene nanoribbon field T effect transistors with engineering the width and geometry of source, channel and drain region in the ballistic regime ⁎ Hossein Fatholah nejada, Daryoosh Didebana,b, , Abbas Ketabib, Mehran Valia, Amir Hossein Bayania, Hadi Heidaric a Institute of Nanoscience and Nanotechnology, University of Kashan, Kashan, Iran b Department of Electrical and Computer Engineering, University of Kashan, Kashan, Iran c Electronics and Nanoscale Engineering Research Division, School of Engineering, University of Glasgow, Glasgow, UK ARTICLE INFO ABSTRACT Keywords: In this paper, with taking advantage of electrical properties of a germanene nanoribbon, we propose a germa- Germanium nene nanoribbon field effect transistor (GeNR-FET). Here by tuning the width and geometry of the germanene Nano-FET nanoribbon in the source, drain and channel regions, we investigate theoretically the transistor characteristics, DFT analog and digital performances of these several different GeNR-FETs at room temperature. Our simulations are NEGF obtained using density functional theory (DFT) combined withnon-equilibrium Green's function (NEGF) method. Ge-NRFET The simulation results show that for digital applications, by tuning the width of the germanene nanoribbon a GeNR-FET with a finite band gap in the channel region and small band gap in the source and drain regions shows a better Ion/Ioff ratio in transfer characteristics. However, for the analog applications, if the band gap of the channel region has small value and the band gap of the source and drain regions have a finite value, the output characteristic shows a higher peak to valley (PVR) ratio which is an important figure of merit in analog ap- plications. -

Exo%C Forms of Low-Dimensional Ar%Ficial Xenes
Exoc forms of low-dimensional ar+ficial Xenes Guy Le Lay Aix-Marseille University, CNRS-PIIM, France Workshop on Anyons in Quantum Many-Body Systems, Dresden, Germany, Jan. 20-25, 2019 D Epitaxial silicene archetype 1D pentasilicene nanoribbons Large area stanene structure on Ag(111) on Ag(110) on Ag(111) Co-workers (recently) Europe T. Angot and E. Salomon, Marseille, France Y. Sassa and coll., Uppsala, Sweden S. Cahangirov, Ankara, Turkey H. Sahin and F. Iyikanat, Izmir, Turkey P. Vogt and coll., Chemnitz, Germany P. De Padova and coll., Rome, Italy, M.E. Davila and J.I. Cerda, Madrid, Spain A. RuBio and coll., Hamburg, Germany Japan J. Yuhara and coll., Nagoya, Japan The Hunt for the Topological QuBit When a TI is coated by an s-wave superconductor (SC), the superconducng vorces are Majorana fermions—they are their own anUparUcles. Exchanging or braiding Majorana vorces, as sketched here, leads to non-abelian stasUcs. Such behavior could form the basis piece of hardware (Majorana QuBit) for topological quantum compung. Xiao-Liang Qi and Shou-Cheng Zhang, Physics Today Jan. 2010, 33 The Challenge: the Hardware QSHE « Experimental synthesis and characterizaon of 2D Topological Insulators remain a major challenge at present, offering outstanding opportuniUes for innovaon and breakthrough. » Kou et al., J. Phys. Chem. Le. 2017, 8, 1905 The way: Nanoarchitectonics, i.e., create atomically controlled ar+ficial structure by design The ar+ficial Xenes What aBout Si, Ge, Sn, and Pb group IV arficial counterparts of graphene? XXXXXXXXXXXXXXXXXXXXX Graphene Flat No band gap Minute SOC Silicene Germanene Increasing Spin Orbit Coupling Stanene 82 Plumbene Pb The hardware beyond graphene First predic+on in 1994, 10 years Before the isola+on of graphene! “Theorecal PossiBility of Stage Corrugaon in Si and Ge Analogs of graphite” ⇒ K.