Tuberculosis Prevention, Diagnosis, and Care in Manitoba, 2008-2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toman's Tuberculosis Case Detection, Treatment, and Monitoring
TOMAN’S TUBERCULOSIS TOMAN’S TUBERCULOSIS CASE DETECTION, TREATMENT, AND MONITORING The second edition of this practical, authoritative reference book provides a rational basis for the diagnosis and management of tuberculosis. Written by a number of experts in the field, it remains faithful to Kurt Toman’s original question-and-answer format, with subject matter grouped under the three headings Case detection, Treatment, and Monitoring. It is a testament to the enduring nature of the first edition that so much CASE DETECTION, TREA material has been retained unchanged. At the same time, the new edition has had not only to address the huge resurgence of tuber- culosis, the emergence of multidrug-resistant bacilli, and the special needs of HIV-infected individuals with tuberculosis, but also to encompass significant scientific advances. These changes in the profile of the disease and in approaches to management have inevitably prompted many new questions and answers and given a different complexion to others. Toman’s Tuberculosis remains essential reading for all who need to AND MONITORING TMENT, QUESTIONS learn more about every aspect of tuberculosis – case-finding, manage- ment, and effective control strategies. It provides invaluable support AND to anyone in the front line of the battle against this disease, from ANSWERS programme managers to policy-makers and from medical personnel to volunteer health workers. SECOND EDITION ISBN 92 4 154603 4 WORLD HEALTH ORGANIZATION WHO GENEVA Toman’s Tuberculosis Case detection, treatment, and monitoring – questions and answers SECOND EDITION Edited by T. Frieden WORLD HEALTH ORGANIZATION GENEVA 2004 WHO Library Cataloguing-in-Publication Data Toman’s tuberculosis case detection, treatment, and monitoring : questions and answers / edited by T. -
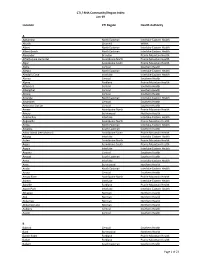
CTI / RHA Community/Region Index Jan-19
CTI / RHA Community/Region Index Jan-19 Location CTI Region Health Authority A Aghaming North Eastman Interlake-Eastern Health Akudik Churchill WRHA Albert North Eastman Interlake-Eastern Health Albert Beach North Eastman Interlake-Eastern Health Alexander Brandon Prairie Mountain Health Alfretta (see Hamiota) Assiniboine North Prairie Mountain Health Algar Assiniboine South Prairie Mountain Health Alpha Central Southern Health Allegra North Eastman Interlake-Eastern Health Almdal's Cove Interlake Interlake-Eastern Health Alonsa Central Southern Health Alpine Parkland Prairie Mountain Health Altamont Central Southern Health Albergthal Central Southern Health Altona Central Southern Health Amanda North Eastman Interlake-Eastern Health Amaranth Central Southern Health Ambroise Station Central Southern Health Ameer Assiniboine North Prairie Mountain Health Amery Burntwood Northern Health Anama Bay Interlake Interlake-Eastern Health Angusville Assiniboine North Prairie Mountain Health Anola North Eastman Interlake-Eastern Health Arbakka South Eastman Southern Health Arbor Island (see Morton) Assiniboine South Prairie Mountain Health Arborg Interlake Interlake-Eastern Health Arden Assiniboine North Prairie Mountain Health Argue Assiniboine South Prairie Mountain Health Argyle Interlake Interlake-Eastern Health Arizona Central Southern Health Amaud South Eastman Southern Health Ames Interlake Interlake-Eastern Health Amot Burntwood Northern Health Anola North Eastman Interlake-Eastern Health Arona Central Southern Health Arrow River Assiniboine -

COVID-19 Community Bulletin #1 Mental Wellness Supports During the COVID-19 Pandemic
COVID-19 Community Bulletin #1 Mental wellness supports during the COVID-19 pandemic Manitoba Keewatinowi Okimakanak (MKO) and Keewatinohk Inniniw Minoayawin Inc. (KIM) are collaborating with Mental Wellness Services in Manitoba to support Northern First Nations’ leadership and Health Directors during the COVID-19 global pandemic. Feelings of distress, anxiety, fear, and grief can heighten as Manitoba communities practice social and physical distancing during this unprecedented health crisis. In response to the need for people to access mental wellness support and service during COVID-19, mental wellness teams and programs have adapted their methods of communication and will respond through virtual means to continue serving those coping with suicide attempts, completed suicides, homicide, multiple deaths, trauma due to violent assault, or other serious events that impact many people. Each Wellness Team is committed to: • Providing confidential mental wellness support with a culturally safe and trauma-informed care approach to all Manitoba First Nations on and off reserve. • Ensuring all services and on-call crisis responses are accessible via telephone or text with various services, including virtual support with FaceTime and/or Zoom video conferencing, where applicable. • Ensuring their mental wellness team members and health care providers are trained to help manage an individual's mental health during COVID-19. • Sharing the most current and accurate information-based facts from provincial and federal public health authorities. • Staying informed of safety measures during COVID-19, as guided by the Province of Manitoba Chief Public Health Officer and public health authorities. COVID-19 Community Bulletin #1 for Leadership & Health Directors – April 7, 2020 Mental Wellness Supports in Manitoba Dakota Ojibway Health Services • Available since 2017, the Dakota Ojibway Tribal Council (DOTC) based in Headingley, provides an on-call crisis response for youth and adults who are in crisis due to mental health concerns, suicide and/or addiction issues. -

Directory – Indigenous Organizations in Manitoba
Indigenous Organizations in Manitoba A directory of groups and programs organized by or for First Nations, Inuit and Metis people Community Development Corporation Manual I 1 INDIGENOUS ORGANIZATIONS IN MANITOBA A Directory of Groups and Programs Organized by or for First Nations, Inuit and Metis People Compiled, edited and printed by Indigenous Inclusion Directorate Manitoba Education and Training and Indigenous Relations Manitoba Indigenous and Municipal Relations ________________________________________________________________ INTRODUCTION The directory of Indigenous organizations is designed as a useful reference and resource book to help people locate appropriate organizations and services. The directory also serves as a means of improving communications among people. The idea for the directory arose from the desire to make information about Indigenous organizations more available to the public. This directory was first published in 1975 and has grown from 16 pages in the first edition to more than 100 pages in the current edition. The directory reflects the vitality and diversity of Indigenous cultural traditions, organizations, and enterprises. The editorial committee has made every effort to present accurate and up-to-date listings, with fax numbers, email addresses and websites included whenever possible. If you see any errors or omissions, or if you have updated information on any of the programs and services included in this directory, please call, fax or write to the Indigenous Relations, using the contact information on the -

Section M: Community Support
Section M: Community Support Page 251 of 653 Community Support Health Canada’s Regional Advisor for Children Special Services has developed the Children’s Services Reference Chart for general information on what types of health services are available in the First Nations’ communities. Colour coding was used to indicate where similar services might be accessible from the various community programs. A legend that explains each of the colours /categories can be found in the centre of chart. By using the chart’s colour coding system, resource teachers may be able to contact the communities’ agencies and begin to open new lines of communication in order to create opportunities for cost sharing for special needs services with the schools. However, it needs to be noted that not all First Nations’ communities offer the depth or variety of the services described due to many factors (i.e., budgets). Unfortunately, there are times when special needs services are required but cannot be accessed for reasons beyond the school and community. It is then that resource teachers should contact Manitoba’s Regional Advisor for Children Special Services to ask for direction and assistance in resolving the issue. Manitoba’s Regional Advisor, Children’s Special Services, First Nations and Inuit Health Programs is Mary L. Brown. Phone: 204-‐983-‐1613 Fax: 204-‐983-‐0079 Email: [email protected] On page two is the Children’s Services Reference Chart and on the following page is information from the chart in a clearer and more readable format including -

Regional Stakeholders in Resource Development Or Protection of Human Health
REGIONAL STAKEHOLDERS IN RESOURCE DEVELOPMENT OR PROTECTION OF HUMAN HEALTH In this section: First Nations and First Nations Organizations ...................................................... 1 Tribal Council Environmental Health Officers (EHO’s) ......................................... 8 Government Agencies with Roles in Human Health .......................................... 10 Health Canada Environmental Health Officers – Manitoba Region .................... 14 Manitoba Government Departments and Branches .......................................... 16 Industrial Permits and Licensing ........................................................................ 16 Active Large Industrial and Commercial Companies by Sector........................... 23 Agricultural Organizations ................................................................................ 31 Workplace Safety .............................................................................................. 39 Governmental and Non-Governmental Environmental Organizations ............... 41 First Nations and First Nations Organizations 1 | P a g e REGIONAL STAKEHOLDERS FIRST NATIONS AND FIRST NATIONS ORGANIZATIONS Berens River First Nation Box 343, Berens River, MB R0B 0A0 Phone: 204-382-2265 Birdtail Sioux First Nation Box 131, Beulah, MB R0H 0B0 Phone: 204-568-4545 Black River First Nation Box 220, O’Hanley, MB R0E 1K0 Phone: 204-367-8089 Bloodvein First Nation General Delivery, Bloodvein, MB R0C 0J0 Phone: 204-395-2161 Brochet (Barrens Land) First Nation General Delivery, -

PDF File of the Survey from Here
Manitoba Keewatinowi Okimakanak Inc. Survey for MKO Youth Ages 12 to 18 Manitoba Keewatinowi Okimakanak (MKO) is seeking direction from youth from MKO communities on possible programming to support you during and after the COVID-19 pandemic. Please complete the following survey by March 16, 2021, to be eligible to win a $50 gift card from MKO. There are 10 chances to win! You need to include your contact information at the end of the survey to be eligible to win. We are keeping your name and contact information confidential. While we will publicly share information we learn from the surveys, we will NOT let anyone know the names or contact information of young people who completed the survey. To be eligible to win a gift card, you must be between 12 to 18 years old and from one of our 26 MKO communities. You need to complete the entire survey to be eligible to win the prize. Which MKO community are you from? Please select one: o I am NOT a member of an MKO community o Barren Lands First Nation, Brochet o Bunibonibee Cree Nation, Oxford House o Chemawawin Cree Nation, Easterville o Fox Lake Cree Nation, Gillam o God’s Lake First Nation, God’s Lake Narrows o Granville Lake First Nation, Leaf Rapids o Manto Sipi Cree Nation, God’s River o Marcel Columb First Nation, Lynn Lake o Mathias Colomb Cree Nation, Pukatawagan o Misipawistik Cree Nation, Grand Rapids o Mosakahiken First Nation, Moose Lake o Nisichawayasihk Cree Nation, Nelson House o Northlands First Nation, Lac Brochet o Norway House Cree Nation o Opaskwayak Cree Nation o O-Pipon-Na-Piwin Cree Nation, South Indian Lake o Pimicikamak Cree Nation, Cross Lake o Sapotaweyak Cree Nation, Pelican Rapids o Sayisi Dene Denesuline Nation, Tadoule Lake o Shamattawa First Nation o Sherridon First Nation o Tataskweyak Cree Nation, Split Lake o York Factory First Nation, York Landing o War Lake First Nation, Ilford o White Water Reserve, Saskatchewan o Wuskwi Sipihk Cree Nation **Note, this survey is for MKO community members only. -

World Bank Document
Document of The World Bank Public Disclosure Authorized Report No. 15894-IN STAFF APPRAISAL REPORT INDIA Public Disclosure Authorized PROPOSED TUBERCULOSIS CONTROL PROJECT January 6, 1997 Public Disclosure Authorized Public Disclosure Authorized South Asia Country Department II (Bhutan, India and Nepal) Population and Huaman Resources Operations Division CURRENCY EQUIVALENTS (As of July 1996) Currency Unit = Rupee Rupee 34.70 =US$ 1.00 Rupee 1.0 US$ 0.0288 METRIC EQUIVALENTS 1 Meter (m) 3.28 Feet (ft) 1 Kilometer 0.62 Miles GOVERNMENT FISCAL YEAR April 1 - March 31 ABBREVIATIONS AND ACRONYMS ADGHS Assistant Director General of Health Services AFB Acid Fast Bacilli AIDS Acquired Immunodeficiency Syndrome ARI Annual Risk of Infection BCG Bacilli of Calmette and Guerin BRS Basic Radiological System CDC Centers for Disease Control and Prevention CHC Community Health Center CHEB Central Health Education Bureau DALYs Disability Adjusted Life Years DANIDA Danish Development Agency DDG Deputy Director General DGHS Director General of Health Services DHO District Health (Medical) Officer DOT Directly-Observed Therapy DTC District Tuberculosis Center DTO District Tuberculosis Officer E Ethambutol GOI Government of India H or INH Isoniazid - ii - ABBREVIATIONS AND ACRONYMS (Continued) HIV Human Immunodeficiency Virus IDA International Development Agency IMA Indian Medical Association LCC Long Course Chemotherapy LSHTM London School of Hygiene and Tropical Medicine MIS Management Information System MO Medical Officer MOHFW Ministry of Health and -

Indigenous Engagement
Indigenous Engagement Canada AECOM’s Integrated Our Guiding Who We Are Lifecycle of Services Principles AECOM’s global Indigenous engagement team is managed by Indigenous people. In Canada, − Community influence and input into siting, design, our staff have a long history of working on the and project planning Our Guiding Principles are modeled after the four principles of the front lines of engagement for various levels − Employment and training opportunities of government, industry, and Indigenous medicine wheel: − Retention of community members and communities. Using our extensive engagement Indigenous-owned businesses to assist expertise, we help our client understand the with projects North | mental is THINKING nuances of the negotiation processes, current Pre-planning Empower project participants with a positive and evolving legal context and Indigenous − Capacity building for longer-term project planning contribution in the decision-making process by community issues – and can help make the obtaining critical feedback on project concerns, Duty to Consult and Accommodate a positive alternatives, decisions, and the development of experience. Win-win solutions are our business, Successful Project Planning best practices as is getting it right the first time. We recognize the importance of developing meaningful and Implementation East | spiritual is SEEING relationships based on mutual respect and − Align with free, prior and informed consent Construction & understanding to help to ensure that the work principles Decommissioning Design -

The Roles of Stochasticity and Life History in the Evolutionary
Research Collection Doctoral Thesis Within-host population dynamics and the evolution of drug resistance in bacterial infections Author(s): Cadosch, Dominique Richard Publication Date: 2016 Permanent Link: https://doi.org/10.3929/ethz-a-010795126 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library DISS. ETH NO. 23499 WITHIN-HOSTPOPULATIONDYNAMICSANDTHEEVOLUTION OFDRUGRESISTANCEINBACTERIALINFECTIONS A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZÜRICH (Dr. sc. ETH Zürich) presented by DOMINIQUERICHARDCADOSCH M.Sc. ETH Zürich, Switzerland born on 30.05.1984 citizen of Vaz/Obervaz GR, Switzerland accepted on the recommendation by Prof. Dr. Sebastian Bonhoeffer, examiner Prof. Dr. Theodore H. Cohen, co-examiner PD Dr. Roland Regoes, co-examiner 2016 To my parents, Ruth and Edgar Cadosch, for their sedulous support and guidance. “I have a friend who’s an artist and has sometimes taken a view which I don’t agree with very well. He’ll hold up a flower and say "look how beautiful it is," and I’ll agree. Then he says "I as an artist can see how beautiful this is but you as a scientist take this all apart and it becomes a dull thing," and I think that he’s kind of nutty. First of all, the beauty that he sees is available to other people and to me too, I believe. Although I may not be quite as refined aesthetically as he is ... I can appreciate the beauty of a flower. -

First Nations "! Lake Wasagamack P the Pas ! (# 297) Wasagamack First Nationp! ! ! Mosakahiken Cree Nation P! (# 299) Moose Lake St
102° W 99° W 96° W 93° W 90° W Tatinnai Lake FFiirrsstt NNaattiioonnss N NUNAVUT MMaanniittoobbaa N ° ° 0 0 6 6 Baralzon Lake Nueltin Kasmere Lake Lake Shannon Lake Nejanilini Lake Egenolf Munroe Lake Bain Lake Lake SASKATCHEWAN Northlands Denesuline First Nation (# 317) Shethanei Lake ! ! Sayisi Dene ! Churchill Lac Lac Brochet First Nation Brochet Tadoule (# 303) Lake Hudson Bay r e iv Barren Lands R (# 308) North ! Brochet Knife Lake l Big Sand il Etawney h Lake rc u Lake h C Buckland MANITOBA Lake Northern Southern Indian Lake Indian Lake N N ° ° 7 7 5 Barrington 5 Lake Gauer Lake Lynn Lake er ! Riv ! South Indian Lake n Marcel Colomb First Nation ! o O-Pipon-Na-Piwin Cree Nation ls (# 328) e (# 318) N Waskaiowaka Lake r Fox Lake e Granville Baldock v ! (# 305) i Lake Lake ! R s Leaf Rapids e Gillam y Tataskweyak Cree Nation a P! H (# 306) Rat P! War Lake Lake Split Lake First Nation (# 323) Shamattawa ! ! York Factory ! First Nation Mathias Colomb Ilford First Nation (# 304) York (# 307) (# 311) Landing ! P! Pukatawagan Shamattawa Nelson House P!" Thompson Nisichawayasihk " Cree Nation Partridge Crop (# 313) Lake Burntwood Lake Landing Lake Kississing Lake Atik Lake Setting Sipiwesk Semmens Lake Lake Lake Bunibonibee Cree Nation Snow Lake (# 301) Flin Flon ! P! Manto Sipi Cree Nation P! Oxford Oxford House (# 302) Reed Lake ! Lake Wekisko Lake Walker Lake ! God's ! Cross Lake Band of Indians God's Lake First Nation Lake (# 276) (# 296) !P Lawford Gods Lake Cormorant Hargrave Lake Lake Lake Narrows Molson Lake Red Sucker Lake N N (# 300) ° ° 4 ! Red Sucker Lake ! 4 Beaver 5 Hill Lake 5 Opaskwayak Cree Nation Norway House Cree Nation (# 315) Norway House P!! (# 278) Stevenson Garden Hill First Nations "! Lake Wasagamack P The Pas ! (# 297) Wasagamack First NationP! ! ! Mosakahiken Cree Nation P! (# 299) Moose Lake St. -

TB Notes2000
TB Notes 2000 Think TB! Christmas Seal Campaign, 1945. Recognize possible signs and symptoms of tuberculosis. Early diagnosis and treatment reduces spread. Tent colony for TB patients, TB sanitorium, TB cases reported annually in the U.S. from Colorado Springs, Colorado. 1953, the first year of national TB surveillance. Community x-ray screening campaign. Dr. Robert Koch, discoverer of the tubercle Drugs used to treat TB disease. From left to right: bacillus, the cause of TB. isoniazid, rifampin, pyrazinamide, and ethambutol. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service __________________________________________________________________________________________________ Centers for Disease Control and Prevention (CDC) Atlanta GA 30333 TB Notes No. 1, 2000 Dear Colleague: This has been planned as a special issue to commemorate “TB Control at the Millennium.” We wanted to take the occasion to note what has been accomplished in TB control over the years, and to look forward to the challenges of the future. Our focus is mainly on progress made in this country, since that is our charge and our responsibility, but one cannot talk about TB control without talking about the important work of researchers and workers in other countries, and therefore we have included articles about international efforts as well. In this issue you will find information about the history of the interaction between mankind and Mycobacterium tuberculosis; the many accomplishments of our partners in TB contol; the past and present activities of the Division of TB Elimination (DTBE); a timeline of some of the highlights of TB control over the past century; educational materials available from DTBE; and photos of historical people, places, events, and memorabilia that we believe you will find interesting.