Severe Systemic Toxicity Caused by Brimonidine Drops in an Infant With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intraocular Pressure Rise After Phacoemulsification Prophylactic
British Journal of Ophthalmology 1995; 79: 809-813 809 Intraocular pressure rise after phacoemulsification Br J Ophthalmol: first published as 10.1136/bjo.79.9.809 on 1 September 1995. Downloaded from with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience Thomas G Bomer, Wolf-Dietrich A Lagreze, Jens Funk Abstract pressure rises usually occur between 6 and 8 Aims-A prospective clinical trial was hours after surgery.6 carried out to evaluate the effect of Various antiglaucomatous agents have been prophylactic medication, the technique of used to prevent the intraocular pressure rise wound closure, and the surgeon's experi- after cataract extraction. Oral acetazolamide15 16 ence on the intraocular pressure rise after and topical timolol'6-19 lowered the pressure cataract extraction. rise in the early period after intracapsular and Methods-In 100 eyes, the intraocular extracapsular cataract extraction. Levobunolol pressure was measured before as well as proved to be superior to timolol 4-7 hours 2-4, 5-7, and 22-24 hours after phaco- after extracapsular cataract extraction.20 Apra- emulsification and posterior chamber lens clonidine lowered the intraocular pressure rise implantation. Each of 25 patients received after uncomplicated phacoemulsification21 and either 1% topical apraclonidine, 0.5%/o extracapsular cataract extraction22 23 when topical levobunolol, 500 mg oral acetazo- given 30 minutes to 1 hour before surgery, lamide, or placebo. Forty four eyes were whereas immediate postoperative treatment operated with sclerocorneal sutureless with apraclonidine was ineffective.22 24 Miotics tunnel and 56 eyes with corneoscleral are frequently used to promote miosis and incision and suture. -

Brimonidine Tartrate; Brinzolamide
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate ; Brinzolamide This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate; Brinzolamide Dosage Form; Route: Suspension/drops; ophthalmic Strength: 0.2%; 1% Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm, in vivo Strength: 0.2%; 1% Subjects: Males and females with chronic open angle glaucoma or ocular hypertension in both eyes. Additional comments: Specific recommendations are provided below. ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open angle glaucoma and ocular hypertension comparing the test product to the reference listed drug (RLD), each applied as one drop in both eyes three times daily at approximately 8:00 a.m., 4:00 p.m., and 10:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open angle glaucoma or ocular hypertension in both eyes b. -

Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg
OPTOMETRIC STUDY CENTER Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg. % Product Sizes Side Effects Warnings Reduction CHOLINERGIC AGENTS Direct Pilocarpine (generic) Pilocarpine 1%, 2%, 4% Increases trabecular outflow BID-QID/15-25% 15ml Headache, blurred vision, myopia, retinal detachment, bronchiole constriction, Angle closure, shortness of breath, retinal narrowing of angle detachment Indirect Phospholine Iodide (Pfizer) Echothiophate iodide 0.125% Increases trabecular outflow QD-BID/15-25% 5ml Same as above plus cataractogenic iris cysts in children, pupillary block, Same as above, plus avoid prior to any increased paralysis with succinylcholine general anesthetic procedure ALPHA-2 AGONISTS Alphagan P (Allergan) Brimonidine tartrate 0.1%, 0.15% with Purite Decreases aqueous production, increases BID-TID/up to 26% 5ml, 10ml, 15ml Dry mouth, hypotension, bradycardia, follicular conjunctivitis, ocular irritation, Monitor for shortness of breath, dizziness, preservative uveoscleral outflow pruritus, dermatitis, conjunctival blanching, eyelid retraction, mydriasis, drug ocular redness and itching, fatigue allergy Brimonidine tartrate Brimonidine tartrate 0.15%, 0.2% Same as above Same as above 5ml, 10ml Same as above Same as above (generic) Iopidine (Novartis) Apraclonidine 0.5% Decreases aqueous production BID-TID/up to 25% 5ml, 10ml Same as above but higher drug allergy (40%) Same as above BETA-BLOCKERS Non-selective Betagan (Allergan) Levobunolol 0.25%, 0.5% Decreases -

NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (Apraclonidine Hydrochloride) Eye Drops 0.5%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (apraclonidine hydrochloride) Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of Iopidine Eye Drops 0.5% contains apraclonidine hydrochloride 5.75 mg, equivalent to apraclonidine base 5 mg. Excipient with known effect Benzalkonium chloride 0.1 mg per 1 mL as a preservative. For the full list of excipients, see section 6.1. 2. PHARMACEUTICAL FORM Eye drops, solution, sterile, isotonic. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Iopidine Eye Drops 0.5% are indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Iopidine Eye Drops 0.5% to delay surgery should have frequent follow up examinations and treatment should be discontinued if the intraocular pressure rises significantly. The addition of Iopidine Eye Drops 0.5% to patients already using two aqueous suppressing drugs (i.e. beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because apraclonidine is an aqueous-suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP. The IOP-lowering efficacy of Iopidine Eye Drops 0.5% diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month. 4.2. Dose and method of administration Dose One drop of Iopidine Eye Drops 0.5% should be instilled into the affected eye(s) three times per day. -

ALPHAGAN® 0.2% Safely and Effectively
NDA 20613/S-031 Page 4 HIGHLIGHTS OF PRESCRIBING INFORMATION ___________ ___________ ADVERSE REACTIONS Most common adverse reactions occurring in These highlights do not include all the information needed approximately 10 to 30% of patients receiving brimonidine to use ALPHAGAN® 0.2% safely and effectively. See full ophthalmic solution 0.2% included oral dryness, ocular prescribing information for ALPHAGAN® 0.2%. hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival ALPHAGAN® (brimonidine tartrate ophthalmic solution) follicles, ocular allergic reactions, and ocular pruritus. (6.1) 0.2% For Topical Ophthalmic Use To report SUSPECTED ADVERSE REACTIONS, Initial U.S. Approval: 1996 contact Allergan at 1-800-433-8871 or the FDA at 1 800-FDA-1088 or www.fda.gov/medwatch. _________ _________ INDICATIONS AND USAGE ___________ ____________ DRUG INTERACTIONS ALPHAGAN® 0.2% is an alpha adrenergic agonist indicated Antihypertensives/cardiac glycosides may lower blood for lowering intraocular pressure (IOP) in patients with open- pressure. (7.1) angle glaucoma or ocular hypertension. (1) ________ ________ Use with CNS depressants may result in an additive or DOSAGE AND ADMINISTRATION potentiating effect. (7.2) One drop in the affected eye(s), three times daily, Tricyclic antidepressants may potentially blunt the approximately 8 hours apart. (2) hypotensive effect of systemic clonidine. (7.3) _______ _______ DOSAGE FORMS AND STRENGTHS Monoamine oxidase inhibitors may result in increased Solution containing 2 mg/mL brimonidine tartrate. (3) hypotension. (7.4) _________ _____________ _______ ______ CONTRAINDICATIONS USE IN SPECIFIC POPULATIONS Neonates and infants (under the age of 2 years). (4.1) Use with caution in children > 2 years of age. -

New Medicines Committee Briefing March 2015 Simbrinza
New Medicines Committee Briefing March 2015 Simbrinza® (Brinzolamide 10mg + Brimonidine tartrate 2mg/ml) for treatment of elevated intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension. Simbrinza® is to be reviewed for use within: Primary Care Secondary Care Summary: Simbrinza® is a combination of brinzolamide which is a carbonic anhydrase (CA-II) inhibitor and brimonidine which is an alpha-2 adrenergic agonist. Simbrinza® is licensed for the treatment of elevated IOP in adult patients with open-angle glaucoma or ocular hypertension whom failed on mono-therapy. Simbrinza® is administered twice daily in the affected eye(s). Simbrinza® is the first combination therapy which does not contain a beta-blocker. SMC has accepted Simbrinza® for use within NHS Scotland, as there is no significant additional cost associated with the combination product compared with its individual components. One trial indicated that fixed combination of Simbrinza® administered BD had a significantly greater IOP lowering effect compared to either brinzolamide 1% or brimonidine 0.2% alone and displayed a safety profile consistent with its individual components. Another trial noted Simbrinza® to be non-inferior to separate administration of brimonidine and brinzolamide less than 10 min apart to prevent wash out. 1 Formulary application: Opthamology department: Consultant submitting application: Mr Lynval Jones (Consultant Ophthalmologist, Glaucoma) Clinical Director supporting application: Gareth Rowland (Clinical Director of Specialised Surgery) Mr Jones has requested for Simbrinza® to be considered for inclusion in the North Staffordshire Joint Formulary for the treatment of elevated intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension who have not responded to monotherapy. -

View Board, Austin, Texas, As Well As the Ethics Com- Pendent Examiner in the Same Room with the Same Instru- Mittee of Eye Center and Research, Aseer, Saudi Arabia
Abdelkader and Kaufman Eye and Vision (2016) 3:31 DOI 10.1186/s40662-016-0065-3 RESEARCH Open Access Clinical outcomes of combined versus separate carbachol and brimonidine drops in correcting presbyopia Almamoun Abdelkader1* and Herbert E. Kaufman2 Abstract Background: To test and compare in a masked fashion the efficacy of using a parasympathomimetic drug (3% carbachol) and an alpha-2 agonist (0.2% brimonidine) in both combined and separate forms to create optically beneficial miosis to pharmacologically improve vision in presbyopia. Methods: A prospective, double-masked, randomized, controlled clinical trial was conducted. Ten naturally emmetropic and presbyopic subjects between 42 and 58 years old with uncorrected distance visual acuity of at least 20/20 in both eyes without additional ocular pathology were eligible for inclusion. All subjects received 3% carbachol and 0.2% brimonidine in both combined and separate forms, 3% carbachol alone and 0.2% brimonidine (control) alone in their non-dominant eye in a crossover manner with one week washout between tests. The subjects’ pupil sizes and both near and distance visual acuities will be evaluated pre- and post-treatment at 1, 2, 4, and 8 h, by a masked examiner at the same room illumination. Results: Statistically significant improvement in mean near visual acuity (NVA) was achieved in all subjects who received combined 3% carbachol and 0.2% brimonidine in the same formula compared with those who received separate forms or carbachol alone or brimonidine alone (P < 0.0001). Conclusion: Based on the data, the combined solution demonstrated greater efficacy than the other solutions that were tested. -

WO 2014/066775 Al 1 May 2014 (01.05.2014) W P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/066775 Al 1 May 2014 (01.05.2014) W P O PCT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61F 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 13/066834 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 25 October 2013 (25.10.201 3) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 61/719,144 26 October 2012 (26. 10.2012) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: FORSIGHT VISION5, INC. [US/US]; 191 kind of regional protection available): ARIPO (BW, GH, Jefferson Drive, Menlo Park, CA 94025 (US). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: RUBIN, Anne, Brody; 191 Jefferson Drive, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Menlo Park, CA 94025 (US). -

Hyperlinked Region IX SOP for MWLCEMS
2 Region IX 2 0 0 STANDARD OPERATING PROCEDURES/ 1 1 STANDING MEDICAL ORDERS 9 9 McHenry Western Lake County EMS System Healthcare delivery requires structure (people, equipment, education) and process (policies, protocols, procedures) that, when integrated, produce a system (programs, organizations, cultures) that leads to optimal outcomes (patient survival and safety, quality, satisfaction). An effective system of care comprises all of these elements—structure, process, system, and patient outcomes—in a framework of continuous quality improvement (AHA, 2015). These protocols have been developed and approved through a collaborative process involving the Advocate Lutheran General; Greater Elgin Area, McHenry Western Lake County, Northwest Community, Amita Saint Joseph Hospital, and Southern Fox Valley EMS Systems to reduce variation in practice and establish a Region-wide System of care. They shall be used: as the written practice guidelines/pathways of care approved by the EMS Medical Directors (EMS MDs) to be initiated by System EMS personnel for off-line medical control. as the standing medical orders to be used by Emergency Communications Registered Nurses (ECRNs) when providing on-line medical control (OLMC). in medium to large scale multiple patient incidents, given that the usual and customary forms of communication are contraindicated as specified in the Region IX disaster plan. System members are authorized to implement these orders to their scope of practice. OLMC communication shall be established without endangering the patient. Under no circumstances shall emergency prehospital care be delayed while attempting to establish contact with a hospital. In the event that communications cannot be established, EMS personnel shall continue to provide care to the degree authorized by their license, these protocols, drugs/equipment available, and their scope of practice granted by the EMS MD in that System. -

0Bcore Safety Profile
Core Safety Profile Active substance: Levobunolol Pharmaceutical form(s)/strength: Eye drops solution/ 0,1%; 0,25%; 0,5%; 0,5% UD P-RMS: CZ/H/PSUR/0006/001 Date of FAR: 26.05.2009 4.2 Posology and method of administration Adults (including the elderly) Country specific posology and method of administration to be included. Children /.../ is not recommended for use in children due to lack of safety and efficacy data. If required, /.../ may be used with other agents to lower intra-ocular pressure. The use of two topical beta-adrenergic blocking agents is not recommended (see section 4.4). Intraocular pressure should be measured approximately four weeks after starting treatment with /.../ as a return to normal ocular pressure can take a few weeks. As with any eye drops, to reduce possible systemic absorption, it is recommended that the lachrymal sac is compressed at the medial canthus (punctual occlusion) for one minute. This should be performed immediately following the instillation of each drop. Transfer from other beta-blocking treatment When another beta blocking agent is being used treatment must be discontinued after a full day of therapy. Start treatment with /.../ the next day with X drop of /.../ topically applied into the conjunctival sac in the affected eye(s). If /.../ is to replace a combination of anti-glaucoma products, only a single product should be removed at a time. Use in renal and hepatic impairment Levobunolol hydrochloride has not been studied in patients with hepatic or renal impairment. Therefore, caution should be used in treating such patients (see section 4.4). -
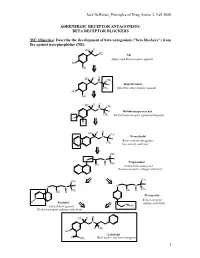
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Generic Drugs and Trade Name Equivalents: Alphabetized by Generic Name
GENERIC DRUGS AND TRADE NAME EQUIVALENTS: ALPHABETIZED BY GENERIC NAME Generic Trade Generic Trade Generic Trade acetaminophen Tylenol escitalopram Lexapro niacin, nicotinic acid Niaspan, Slo-Niacin acetaminophen-codeine Tylenol #3 estrogen Premarin nitroglycerin Nitro-bid acetazolamide Diamox eszopiclone Lunesta nystatin Mycostatin acyclovir Zovirax ethambutol Myambutol ofloxacin Ocuflox albuterol Proventil, Ventolin eye vitamin supplement Ocuvite PreserVision olopatadine Patanol alendronate Fosamax famciclovir Famvir omeprazole Prilosec allopurinol Zyloprim felodipine Plendil paraffin-mineral oil Lacri-Lube alprazolam Xanax fexofenadine Allegra paroxetine Paxil aminocaproic acid Amicar fexofenadine-pseudoephedrine Allegra-D pegaptanib Macugen amiodarone Cordarone finasteride Propecia, Proscar peginterferon alfa-2a Pegasys amitryptyline Elavil fluconazole Diflucan peginterferon alfa-2b PegIntron amlodipine Norvasc flunisolide AeroBid pemirolast potassium Alamast amoxicillin Amoxil, Dispermox fluorometholone FML phenytoin Dilantin amoxicillin-clavulanate Augmentin fluorouracil Efudex pilocarpine IsoptoCarpine, Pilocar apraclonidine Iopidine fluoxetine Prozac piroxicam Feldene AREDS formula PreserVision flutamide Eulexin polymyxin B-bacitracin Polysporin atenolol Tenormin fluticasone Flovent, Flonase polymyxin B-bacitracin-neomycin Neosporin (ointment) atorvastatin Lipitor folinic acid Folixor polymyxin B-neomycin-gramicidin Neosporin (suspension) azathioprine Imuran foscarnet Foscavir potassium chloride Micro-K, K-Dur, Klor-Com azelastine