Equation of State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Non-Local Momentum Transport Parameterizations
Non-local Momentum Transport Parameterizations Joe Tribbia NCAR.ESSL.CGD.AMP Outline • Historical view: gravity wave drag (GWD) and convective momentum transport (CMT) • GWD development -semi-linear theory -impact • CMT development -theory -impact Both parameterizations of recent vintage compared to radiation or PBL GWD CMT • 1960’s discussion by Philips, • 1972 cumulus vorticity Blumen and Bretherton damping ‘observed’ Holton • 1970’s quantification Lilly • 1976 Schneider and and momentum budget by Lindzen -Cumulus Friction Swinbank • 1980’s NASA GLAS model- • 1980’s incorporation into Helfand NWP and climate models- • 1990’s pressure term- Miller and Palmer and Gregory McFarlane Atmospheric Gravity Waves Simple gravity wave model Topographic Gravity Waves and Drag • Flow over topography generates gravity (i.e. buoyancy) waves • <u’w’> is positive in example • Power spectrum of Earth’s topography α k-2 so there is a lot of subgrid orography • Subgrid orography generating unresolved gravity waves can transport momentum vertically • Let’s parameterize this mechanism! Begin with linear wave theory Simplest model for gravity waves: with Assume w’ α ei(kx+mz-σt) gives the dispersion relation or Linear theory (cont.) Sinusoidal topography ; set σ=0. Gives linear lower BC Small scale waves k>N/U0 decay Larger scale waves k<N/U0 propagate Semi-linear Parameterization Propagating solution with upward group velocity In the hydrostatic limit The surface drag can be related to the momentum transport δh=isentropic Momentum transport invariant by displacement Eliassen-Palm. Deposited when η=U linear theory is invalid (CL, breaking) z φ=phase Gravity Wave Drag Parameterization Convective or shear instabilty begins to dissipate wave- momentum flux no longer constant Waves propagate vertically, amplitude grows as r-1/2 (energy force cons.). -
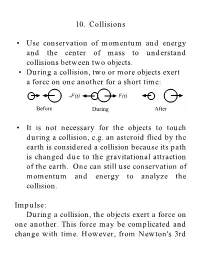
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Thermodynamics, Flame Temperature and Equilibrium
Thermodynamics, Flame Temperature and Equilibrium Combustion Summer School 2018 Prof. Dr.-Ing. Heinz Pitsch Course Overview Part I: Fundamentals and Laminar Flames • Introduction • Fundamentals and mass balances of combustion systems • Thermodynamic quantities • Thermodynamics, flame • Flame temperature at complete temperature, and equilibrium conversion • Governing equations • Chemical equilibrium • Laminar premixed flames: Kinematics and burning velocity • Laminar premixed flames: Flame structure • Laminar diffusion flames • FlameMaster flame calculator 2 Thermodynamic Quantities First law of thermodynamics - balance between different forms of energy • Change of specific internal energy: du specific work due to volumetric changes: δw = -pdv , v=1/ρ specific heat transfer from the surroundings: δq • Related quantities specific enthalpy (general definition): specific enthalpy for an ideal gas: • Energy balance for a closed system: 3 Multicomponent system • Specific internal energy and specific enthalpy of mixtures • Relation between internal energy and enthalpy of single species 4 Multicomponent system • Ideal gas u and h only function of temperature • If cpi is specific heat at constant pressure and hi,ref is reference enthalpy at reference temperature Tref , temperature dependence of partial specific enthalpy is given by • Reference temperature may be arbitrarily chosen, most frequently used: Tref = 0 K or Tref = 298.15 K 5 Multicomponent system • Partial molar enthalpy hi,m is and its temperature dependence is where the molar specific -

Fluids – Lecture 7 Notes 1
Fluids – Lecture 7 Notes 1. Momentum Flow 2. Momentum Conservation Reading: Anderson 2.5 Momentum Flow Before we can apply the principle of momentum conservation to a fixed permeable control volume, we must first examine the effect of flow through its surface. When material flows through the surface, it carries not only mass, but momentum as well. The momentum flow can be described as −→ −→ momentum flow = (mass flow) × (momentum /mass) where the mass flow was defined earlier, and the momentum/mass is simply the velocity vector V~ . Therefore −→ momentum flow =m ˙ V~ = ρ V~ ·nˆ A V~ = ρVnA V~ where Vn = V~ ·nˆ as before. Note that while mass flow is a scalar, the momentum flow is a vector, and points in the same direction as V~ . The momentum flux vector is defined simply as the momentum flow per area. −→ momentum flux = ρVn V~ V n^ . mV . A m ρ Momentum Conservation Newton’s second law states that during a short time interval dt, the impulse of a force F~ applied to some affected mass, will produce a momentum change dP~a in that affected mass. When applied to a fixed control volume, this principle becomes F dP~ a = F~ (1) dt V . dP~ ˙ ˙ P(t) P + P~ − P~ = F~ (2) . out dt out in Pin 1 In the second equation (2), P~ is defined as the instantaneous momentum inside the control volume. P~ (t) ≡ ρ V~ dV ZZZ ˙ The P~ out is added because mass leaving the control volume carries away momentum provided ˙ by F~ , which P~ alone doesn’t account for. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

Leonhard Euler: His Life, the Man, and His Works∗
SIAM REVIEW c 2008 Walter Gautschi Vol. 50, No. 1, pp. 3–33 Leonhard Euler: His Life, the Man, and His Works∗ Walter Gautschi† Abstract. On the occasion of the 300th anniversary (on April 15, 2007) of Euler’s birth, an attempt is made to bring Euler’s genius to the attention of a broad segment of the educated public. The three stations of his life—Basel, St. Petersburg, andBerlin—are sketchedandthe principal works identified in more or less chronological order. To convey a flavor of his work andits impact on modernscience, a few of Euler’s memorable contributions are selected anddiscussedinmore detail. Remarks on Euler’s personality, intellect, andcraftsmanship roundout the presentation. Key words. LeonhardEuler, sketch of Euler’s life, works, andpersonality AMS subject classification. 01A50 DOI. 10.1137/070702710 Seh ich die Werke der Meister an, So sehe ich, was sie getan; Betracht ich meine Siebensachen, Seh ich, was ich h¨att sollen machen. –Goethe, Weimar 1814/1815 1. Introduction. It is a virtually impossible task to do justice, in a short span of time and space, to the great genius of Leonhard Euler. All we can do, in this lecture, is to bring across some glimpses of Euler’s incredibly voluminous and diverse work, which today fills 74 massive volumes of the Opera omnia (with two more to come). Nine additional volumes of correspondence are planned and have already appeared in part, and about seven volumes of notebooks and diaries still await editing! We begin in section 2 with a brief outline of Euler’s life, going through the three stations of his life: Basel, St. -

Duality of Momentum-Energy and Space-Time on an Almost Complex
Duality of momentum-energy and space-time on an almost complex manifold You-gang Feng Department of Basic Sciences, College of Science, Guizhou University, Cai-jia Guan, Guiyang, 550003 China Abstract: We proved that under quantum mechanics a momentum-energy and a space-time are dual vector spaces on an almost complex manifold in position representation, and the minimal uncertainty relations are equivalent to the inner-product relations of their bases. In a microscopic sense, there exist locally a momentum-energy conservation and a space-time conservation. The minimal uncertainty relations refer to a local equilibrium state for a stable system, and the relations will be invariable in the special relativity. A supposition about something having dark property is proposed, which relates to a breakdown of time symmetry. Keywords: Duality, Manifold, momentum-energy, space-time, dark PACS (2006): 03.65.Ta 02.40.Tt 95.35.+d 95.36.+x 1. Introduction The uncertainty relations are regarded as a base of quantum mechanics. Understanding this law underwent many changes for people. At fist, it was considered as a measuring theory that there was a restricted relation between a position and a momentum for a particle while both of them were measured. Later on, people further realized that the restricted relation always exists even if there is no measurement. A modern point of view on the law is that it shows a wave-particle duality of a microscopic matter. The importance of the uncertainty relations is rising with the development of science and technology. In the quantum communication theory, which has been rapid in recent years, an uncertainty relation between a particle’s position and a total momentum of a system should be considered while two particles’ 1 entangled states are used for communication [1] . -

Leonhard Euler - Wikipedia, the Free Encyclopedia Page 1 of 14
Leonhard Euler - Wikipedia, the free encyclopedia Page 1 of 14 Leonhard Euler From Wikipedia, the free encyclopedia Leonhard Euler ( German pronunciation: [l]; English Leonhard Euler approximation, "Oiler" [1] 15 April 1707 – 18 September 1783) was a pioneering Swiss mathematician and physicist. He made important discoveries in fields as diverse as infinitesimal calculus and graph theory. He also introduced much of the modern mathematical terminology and notation, particularly for mathematical analysis, such as the notion of a mathematical function.[2] He is also renowned for his work in mechanics, fluid dynamics, optics, and astronomy. Euler spent most of his adult life in St. Petersburg, Russia, and in Berlin, Prussia. He is considered to be the preeminent mathematician of the 18th century, and one of the greatest of all time. He is also one of the most prolific mathematicians ever; his collected works fill 60–80 quarto volumes. [3] A statement attributed to Pierre-Simon Laplace expresses Euler's influence on mathematics: "Read Euler, read Euler, he is our teacher in all things," which has also been translated as "Read Portrait by Emanuel Handmann 1756(?) Euler, read Euler, he is the master of us all." [4] Born 15 April 1707 Euler was featured on the sixth series of the Swiss 10- Basel, Switzerland franc banknote and on numerous Swiss, German, and Died Russian postage stamps. The asteroid 2002 Euler was 18 September 1783 (aged 76) named in his honor. He is also commemorated by the [OS: 7 September 1783] Lutheran Church on their Calendar of Saints on 24 St. Petersburg, Russia May – he was a devout Christian (and believer in Residence Prussia, Russia biblical inerrancy) who wrote apologetics and argued Switzerland [5] forcefully against the prominent atheists of his time. -

Module P7.4 Specific Heat, Latent Heat and Entropy
FLEXIBLE LEARNING APPROACH TO PHYSICS Module P7.4 Specific heat, latent heat and entropy 1 Opening items 4 PVT-surfaces and changes of phase 1.1 Module introduction 4.1 The critical point 1.2 Fast track questions 4.2 The triple point 1.3 Ready to study? 4.3 The Clausius–Clapeyron equation 2 Heating solids and liquids 5 Entropy and the second law of thermodynamics 2.1 Heat, work and internal energy 5.1 The second law of thermodynamics 2.2 Changes of temperature: specific heat 5.2 Entropy: a function of state 2.3 Changes of phase: latent heat 5.3 The principle of entropy increase 2.4 Measuring specific heats and latent heats 5.4 The irreversibility of nature 3 Heating gases 6 Closing items 3.1 Ideal gases 6.1 Module summary 3.2 Principal specific heats: monatomic ideal gases 6.2 Achievements 3.3 Principal specific heats: other gases 6.3 Exit test 3.4 Isothermal and adiabatic processes Exit module FLAP P7.4 Specific heat, latent heat and entropy COPYRIGHT © 1998 THE OPEN UNIVERSITY S570 V1.1 1 Opening items 1.1 Module introduction What happens when a substance is heated? Its temperature may rise; it may melt or evaporate; it may expand and do work1—1the net effect of the heating depends on the conditions under which the heating takes place. In this module we discuss the heating of solids, liquids and gases under a variety of conditions. We also look more generally at the problem of converting heat into useful work, and the related issue of the irreversibility of many natural processes. -

Calculation of Chemical Potential and Activity Coefficient of Two Layers of Co2 Adsorbed on a Graphite Surface
Physical Chemistry Chemical Physics CALCULATION OF CHEMICAL POTENTIAL AND ACTIVITY COEFFICIENT OF TWO LAYERS OF CO2 ADSORBED ON A GRAPHITE SURFACE Journal: Physical Chemistry Chemical Physics Manuscript ID: CP-ART-08-2014-003782.R1 Article Type: Paper Date Submitted by the Author: 07-Nov-2014 Complete List of Authors: Trinh, Thuat; NTNU, Department of Chemistry Bedeaux, Dick; NTNU, Chemistry Simon, Jean-Marc; Universit� de Bourgogne, Chemistry Kjelstrup, Signe; Norwegian University of Science and Technology, Natural Sciences Page 1 of 8 Physical Chemistry Chemical Physics PCCP RSC Publishing ARTICLE CALCULATION OF CHEMICAL POTENTIAL AND ACTIVITY COEFFICIENT OF TWO LAYERS Cite this: DOI: 10.1039/x0xx00000x OF CO2 ADSORBED ON A GRAPHITE SURFACE T.T. Trinh,a D. Bedeaux a , J.-M Simon b and S. Kjelstrup a,c,* Received 00th January 2014, Accepted 00th January 2014 We study the adsorption of carbon dioxide at a graphite surface using the new Small System DOI: 10.1039/x0xx00000x Method, and find that for the temperature range between 300K and 550K most relevant for CO separation; adsorption takes place in two distinct thermodynamic layers defined according www.rsc.org/ 2 to Gibbs. We calculate the chemical potential, activity coefficient in both layers directly from the simulations. Based on thermodynamic relations, the entropy and enthalpy of the CO 2 adsorbed layers are also obtained. Their values indicate that there is a trade-off between entropy and enthalpy when a molecule chooses for one of the two layers. The first layer is a densely packed monolayer of relatively constant excess density with relatively large repulsive interactions and negative enthalpy.