Cellular Changes of the Corneal Epithelium and Stroma in Herpes Simplex Keratitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic and Inducing Effect of Corneal Crosslinking on Infectious
Differenteffectsofcornealcrosslinkingoninfectiouskeratitis 窑Review窑 Therapeuticandinducingeffectofcornealcrosslinking oninfectiouskeratitis 1DepartmentofOphthalmology,ShandongProvincial thecornealintrinsicbiomechanicalpropertyandthestiffness HospitalAffiliatedtoShandongUniversity,Jinan250000, ofcorneatoresistectasiaofcornea [1].Besidesitsoriginal ShandongProvince,China applicationforthekeratoconusandkeratectasia [2],CXLhas 2DepartmentofOphthalmology,thePeople'sHospitalof beenutilizedontothetreatmentofinfectiouskeratitis [3], Linyi,Linyi276000,ShandongProvince,China nowadays.Althoughthesecondaryinfectiouskeratitisafter 3DepartmentofPediatrics,thePeople'sHospitalofLinyi, CXLisrare,therearesomereportsonsecondarykeratitis Linyi276000,ShandongProvince,China infectedby bacteria,fungi,herpessimplexvirusand Co-firstauthors: Liang-ZhuJiangandShi-YanQiu Acanthamoeba.ThisrarecomplicationofCXLcancause Correspondence to: Guo-YingMu.Departmentof seriousocularmorbidityandhaveasubsequentdamaging Ophthalmology,ShandongProvincialHospitalAffiliatedto effectonthepatient'svision.ThesurgicaltechniqueofCXL ShandongUniversity,Jinan250000,ShandongProvince, involvestheremovalofepitheliumintraoperativelyandthe [email protected] applicationofcontactlenspostoperatively.Thesefactors Received:2015-06-30Accepted:2016-08-09 havebeenassociatedwiththeoccurrenceofinfectious keratitisafterCXL.Inpresentstudy,wesummarizedthe Abstract therapeuticeffectofCXLoninfectiouskeratitisandthe · Thecornealcrosslinking (CXL)withriboflavinand keratitissecondarytocorneaCXLreportedbyprevious -

CAUSES, COMPLICATIONS &TREATMENT of A“RED EYE”
CAUSES, COMPLICATIONS & TREATMENT of a “RED EYE” 8 Most cases of “red eye” seen in general practice are likely to be conjunctivitis or a superficial corneal injury, however, red eye can also indicate a serious eye condition such as acute angle glaucoma, iritis, keratitis or scleritis. Features such as significant pain, photophobia, reduced visual acuity and a unilateral presentation are “red flags” that a sight-threatening condition may be present. In the absence of specialised eye examination equipment, such as a slit lamp, General Practitioners must rely on identifying these key features to know which patients require referral to an Ophthalmologist for further assessment. Is it conjunctivitis or is it something more Iritis is also known as anterior uveitis; posterior uveitis is serious? inflammation of the choroid (choroiditis). Complications include glaucoma, cataract and macular oedema. The most likely cause of a red eye in patients who present to 4. Scleritis is inflammation of the sclera. This is a very rare general practice is conjunctivitis. However, red eye can also be presentation, usually associated with autoimmune a feature of a more serious eye condition, in which a delay in disease, e.g. rheumatoid arthritis. treatment due to a missed diagnosis can result in permanent 5. Penetrating eye injury or embedded foreign body; red visual loss. In addition, the inappropriate use of antibacterial eye is not always a feature topical eye preparations contributes to antimicrobial 6. Acid or alkali burn to the eye resistance. The patient history will usually identify a penetrating eye injury Most general practice clinics will not have access to specialised or chemical burn to the eye, but further assessment may be equipment for eye examination, e.g. -

Diagnosis and Treatment of Neurotrophic Keratopathy
An Evidence-based Approach to the Diagnosis and Treatment of Neurotrophic Keratopathy ACTIVITY DIRECTOR A CME MONOGRAPH Esen K. Akpek, MD This monograph was published by Johns Hopkins School of Medicine in partnership Wilmer Eye Institute with Catalyst Medical Education, LLC. It is Johns Hopkins School of Medicine not affiliated with JAMA medical research Baltimore, Maryland publishing. Visit catalystmeded.com/NK for online testing to earn your CME credit. FACULTY Natalie Afshari, MD Mina Massaro-Giordano, MD Shiley Eye Institute University of Pennsylvania School of Medicine University of California, San Diego Philadelphia, Pennsylvania La Jolla, California Nakul Shekhawat, MD, MPH Sumayya Ahmad, MD Wilmer Eye Institute Mount Sinai School of Medicine Johns Hopkins School of Medicine New York, New York Baltimore, Maryland Pedram Hamrah, MD, FRCS, FARVO Christopher E. Starr, MD Tufts University School of Medicine Weill Cornell Medical College Boston, Massachusetts New York, New York ACTIVITY DIRECTOR FACULTY Esen K. Akpek, MD Natalie Afshari, MD Mina Massaro-Giordano, MD Professor of Ophthalmology Professor of Ophthalmology Professor of Clinical Ophthalmology Director, Ocular Surface Diseases Chief of Cornea and Refractive Surgery University of Pennsylvania School and Dry Eye Clinic Vice Chair of Education of Medicine Wilmer Eye Institute Fellowship Program Director of Cornea Philadelphia, Pennsylvania Johns Hopkins School of Medicine and Refractive Surgery Baltimore, Maryland Shiley Eye Institute Nakul Shekhawat, MD, MPH University of California, -

Herpetic Corneal Infections
FocalPoints Clinical Modules for Ophthalmologists VOLUME XXVI, NUMBER 8 SEPTEMBER 2008 (MODULE 2 OF 3) Herpetic Corneal Infections Sonal S. Tuli, MD Reviewers and Contributing Editors Consultants George A. Stern, MD, Editor for Cornea & External Disease James Chodosh, MD, MPH Kristin M. Hammersmith, MD, Basic and Clinical Science Course Faculty, Section 8 Kirk R. Wilhelmus, MD, PhD Christie Morse, MD, Practicing Ophthalmologists Advisory Committee for Education Focal Points Editorial Review Board George A. Stern, MD, Missoula, MT Claiming CME Credit Editor in Chief, Cornea & External Disease Thomas L. Beardsley, MD, Asheville, NC Academy members: To claim Focal Points CME cred- Cataract its, visit the Academy web site and access CME Central (http://www.aao.org/education/cme) to view and print William S. Clifford, MD, Garden City, KS Glaucoma Surgery; Liaison for Practicing Ophthalmologists Advisory your Academy transcript and report CME credit you Committee for Education have earned. You can claim up to two AMA PRA Cate- gory 1 Credits™ per module. This will give you a maxi- Bradley S. Foster, MD, Springfield, MA Retina & Vitreous mum of 24 credits for the 2008 subscription year. CME credit may be claimed for up to three (3) years from Anil D. Patel, MD, Oklahoma City, OK date of issue. Non-Academy members: For assistance Neuro-Ophthalmology please send an e-mail to [email protected] or a Eric P. Purdy, MD, Fort Wayne, IN fax to (415) 561-8575. Oculoplastic, Lacrimal, & Orbital Surgery Steven I. Rosenfeld, MD, FACS, Delray Beach, FL Refractive Surgery, Optics & Refraction C. Gail Summers, MD, Minneapolis, MN Focal Points (ISSN 0891-8260) is published quarterly by the American Academy of Ophthalmology at 655 Beach St., San Francisco, CA 94109-1336. -

Pediatric Pharmacology and Pathology
7/31/2017 In the next 2 hours……. Pediatric Pharmacology and Pathology . Ocular Medications and Children The content of th is COPE Accredited CE activity was prepared independently by Valerie M. Kattouf O.D. without input from members of the optometric community . Brief review of examination techniques/modifications for children The content and format of this course is presented without commercial bias and does not claim superiority of any commercial product or service . Common Presentations of Pediatric Pathology Valerie M. Kattouf O.D., F.A.A.O. Illinois College of Optometry Chief, Pediatric Binocular Vision Service Associate Professor Ocular Medications & Children Ocular Medications & Children . Pediatric systems differ in: . The rules: – drug excretion – birth 2 years old = 1/2 dose kidney is the main site of drug excretion – 2-3 years old = 2/3 dose diminished 2° renal immaturity – > 3 years old = adult dose – biotransformation liver is organ for drug metabolism Impaired 2° enzyme immaturity . If only 50 % is absorbed may be 10x maximum dosage Punctal Occlusion for 3-4 minutes ↓ systemic absorption by 40% Ocular Medications & Children Ocular Medications & Children . Systemic absorption occurs through….. Ocular Meds with strongest potential for pediatric SE : – Mucous membrane of Nasolacrimal Duct 80% of each gtt passing through NLD system is available for rapid systemic absorption by the nasal mucosa – 10 % Phenylephrine – Conjunctiva – Oropharynx – 2 % Epinephrine – Digestive system (if swallowed) Modified by variation in Gastric pH, delayed gastric emptying & intestinal mobility – 1 % Atropine – Skin (2° overflow from conjunctival sac) Greatest in infants – 2 % Cyclopentalate Blood volume of neonate 1/20 adult Therefore absorbed meds are more concentrated at this age – 1 % Prednisone 1 7/31/2017 Ocular Medications & Children Ocular Medications & Children . -

Herpes Simplex Keratitis
Herpes Simplex Keratitis Herpes simplex eye infection is caused by a type of herpes simplex virus. An episode often clears without any permanent problem. However, in some cases the infection causes scarring to the transparent front part of the eye (the cornea). This can lead to permanent loss of vision. Prompt treatment with antiviral eye ointment or drops helps to prevent corneal scarring. Herpes simplex infections There are two types of herpes simplex virus. Type 1 is the usual cause of cold sores around the mouth, and herpes simplex infection in the eye. Type 2 is the usual cause of genital herpes. It rarely causes cold sores or eye infections. Type 1 herpes simplex infections The first time you are infected is called the primary infection. Many people become infected with this virus, often during childhood. The herpes simplex virus can pass through the moist skin that lines the mouth. It is commonly passed on by close contact such as kisses from a family member who has a cold sore. In many people the primary infection does not cause any symptoms, although in some cases symptoms do occur. Following the primary infection, the virus stays with you for life. It stays inactive (dormant) in the root of a nerve in the face (the trigeminal nerve). • In many people, the virus remains permanently inactive and causes no problems. • In some people, the virus activates and multiplies from time to time. Virus particles then travel down the nerve to cause episodes of active infection with symptoms: • In most of these cases, the virus travels down a branch of the nerve to the mouth to cause cold sores. -

Ophthalmic Steroids Reference Number: OH.PHAR.PPA.100 Effective Date: 01/01/2021 Revision Log Last Review Date: 11.20 Line of Business: Medicaid
Clinical Policy: Ophthalmic Agents: Ophthalmic Steroids Reference Number: OH.PHAR.PPA.100 Effective Date: 01/01/2021 Revision Log Last Review Date: 11.20 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description Ophthalmic Agents: Ophthalmic Steroids NO PA REQUIRED “PREFERRED” PA REQUIRED “NON-PREFERRED” DEXAMETHASONE SODIUM PHOSPHATE ALREX® (loteprednol etabonate) DUREZOL® (difluprednate) FLAREX® (fluorometholone acetate) FLUOROMETHOLONE INVELTYS® (loteprednol etabonate) FML FORTE® (fluorometholone) LOTEMAX® (loteprednol etabonate) FML S.O.P. ® (fluorometholone) LOTEMAX® SM (loteprednol etabonate) PRED MILD® (prednisolone acetate) LOTEPREDNOL PREDNISOLONE ACETATE MAXIDEX® (dexamethasone sodium phosphate) PREDNISOLONE SODIUM PHOSPHATE FDA approved indication(s) Alrex is indicated for: • Temporary relief of the signs and symptoms of seasonal allergic conjunctivitis Durezol is indicated for: • Treatment of postoperative ocular pain and postoperative ocular inflammation • Treatment of endogenous anterior uveitis Flarex, fluorometholone, FML Forte, and FML S.O.P are indicated for: • Treatment of corticosteroid-responsive ophthalmic disorders including allergic conjunctivitis, ocular burns or trauma due to corneal injury resulting from chemical, thermal or penetration trauma, giant papillary conjunctivitis (GPC), keratitis, postoperative ocular inflammation, vernal keratoconjunctivitis, and chronic anterior uveitis Inveltys is indicated for: • Treatment of postoperative -

The Outcome of Penetrating Keratoplasty for Corneal Scarring
ORIGINAL ARTICLE The outcome of penetrating keratoplasty for corneal scarring due to herpes simplex keratitis O resultado da ceratoplastia penetrante para cicatriz de córnea consequente à ceratite por herpes simplex YESIM ALTay1, SEMA TAMER1, ABDULLAH SENCER Kaya1, OZGUR BALTA1, AYSE BURCU1, FIRDEVS ORNEK1 ABSTRACT RESUMO Purpose: To determine the outcomes of penetrating keratoplasty (PK) for treatment Objetivo: Determinar os resultados da ceratoplastia penetrante (PK) para o trata- of corneal scarring caused by Herpes simplex virus (HSV) keratitis, and whether mento da cicatriz da córnea consequente à ceratite por Herpes simplex vírus (HSV), the corneal scar type affects treatment outcome. e se o tipo de cicatriz na córnea afeta o resultado cirúrgico. Methods: A retrospective analysis of patients who underwent PK for HSV-related Métodos: Foi realizada análise retrospectiva dos pacientes, submetidos à PK para corneal scarring between January 2008 and July 2011 was performed. The patients a cicatriz da córnea relacionados com o HSV entre janeiro de 2008 e julho de 2011. were categorized into two groups. Group 1 consisted of patients with a quiescent Os pacientes foram divididos em dois grupos. Grupo 1 consistiu de pacientes que herpetic corneal scar and group 2 consisted of patients who developed a corneal tiveram cicatriz corneana herpética quiescente e grupo 2 consistiu de pacientes que descemetocele or perforation secondary to persistent epithelial defects with no desenvolveram descemetocele ou perfuração córnea secundária a defeitos epiteliais active stromal inflammation. The mean follow-up was 21.30 ± 14.59 months. The persistentes sem inflamação estromal ativa. O seguimento médio foi de 21,30 ± 14,59 main parameters evaluated were recurrence of herpetic keratitis, graft rejection, meses. -

Herpes Simplex Epithelial Keratitis and Proposed Treatments
Herpes Simplex Epithelial Keratitis and Proposed Treatments Andrea De Souza, OD Author’s Bio Dr. Andrea De Souza received her Doctor of Optometry Degree in 2012 from the New England College of Optometry in Boston, MA. She continued her optometric training and graduated from the Primary Care and Contact Lens residency at the UC Berkeley School of Optometry in 2013. Today, Dr. De Souza works as a clinical instructor at the UC Berkeley School of Optometry teaching students in the field of ocular disease, contact lens and primary care. ____________________________________________________________________ I. Introduction Herpes simplex virus (HSV) stromal keratitis is the leading infectious cause of corneal blindness in developed nations. In the United States alone, approximately 46,000 cases of HSV ocular infection are diagnosed each year.1 HSV is divided into two categories: type 1 and type 2. HSV-1, which most commonly infects the mouth and eyes, is transmitted through direct contact of skin sores or oral secretions, often via kissing. HSV-2 typically affects the genitals and is most commonly transmitted in adults through sexual contact or via maternal transmission to newborns during childbirth.2 HSV-2 is thought to infect over 500 million people worldwide and approximately 23 million new cases are reported each year; the incidence of HSV-1 infections, however, are even greater.2 More than 80% of individuals carry herpes simplex virus antibodies, however 94% of primary infections are subclinical.3 Most primary initial infections occur between the ages of six months and five years. Once infected, the virus travels along nerves from the skin and mouth to the dorsal root of the trigeminal ganglion, via axoplasmic transport, and lays dormant. -

Ganciclovir Gel—A New Topical Treatment for Herpetic Keratitis
Foster 15/10/08 03:25 Page 52 Anterior Segment Cornea Ganciclovir Gel—A New Topical Treatment for Herpetic Keratitis a report by C Stephen Foster, MD Clinical Professor of Ophthalmology, Harvard Medical School DOI: 10.17925/USOR.2007.03.00.52 Herpes simplex virus (HSV) infection of the eye is a major cause of corneal with fluorescein. The resulting dendritic keratitis is associated with corneal opacity in the US1 and other developed countries.2 Infection with either scarring and decreased corneal sensation.7 Each recurrence of HSK is HSV-1 or HSV-2 is common. Data from the US National Health and Nutrition associated with increased irregular scarring of the cornea and decreased Examination Survey (NHANES) suggest an overall 58% seroprevalence rate corneal sensitivity. Dendritic ulcers occur in approximately 15% of initial for HSV-1—the predominant cause of herpes simplex keratitis2 (HSK)—with episodes of ocular HSV, with disciform stromal keratitis in 2% of initial cases.8 rates in individual ethnic groups as high as 89%.3 A three-month Dendrites can progress to geographic ulcers, a type of large (amoeboid) prospective epidemiological study conducted in France put the incidence of epithelial defect with fimbriated edges. Geographic ulcers may also arise in HSK at 31.5 cases per 100,000 per person-year.4 Of these, 13.2 cases response to corticosteroid therapy, most likely as a consequence of the represented primary infections and 18.3 were recurrences. HSV infection is immunosuppressive effect of these agents.1,9 The situation can worsen with lifelong and recurrent, with the virus becoming latent in sensory nerves after stromal ulceration, and can progress to descemetocel or even frank the original outbreak. -
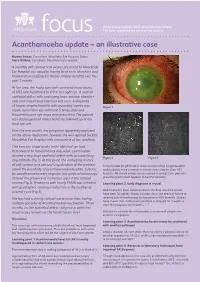
Acanthamoeba Update – an Illustrative Case
An occasional update commissioned by the College. focus The views expressed are those of the authors. Acanthamoeba update – an illustrative case Hamed Anwar, Consultant, Moorfields Eye Hospital, Dubai Mark Wilkins, Consultant, Moorfields Eye Hospital A monthly soft contact lens wearer presented to Moorfields Eye Hospital eye casualty, having been seen elsewhere and treated unsuccessfully for herpes simplex keratitis over the past 1 month. At the time, she had a spectacle corrected visual acuity of 6/12 which pinholed to 6/9 in her right eye. A corneal epithelial defect with underlying haze, anterior chamber cells and conjunctival injection was seen. A diagnosis of herpes simplex keratitis with secondary uveitis was Figure 1 made. Ganciclovir eye ointment 5 times daily and Dexamethasone eye drops were prescribed. The patient was discharged and instructed to be followed up at her local eye unit. Over the next month, her symptoms apparently improved on the above medication, however she was referred back to Moorfields Eye Hospital with a recurrence of her condition. This time the visual acuity in the affected eye had deteriorated to hand motions only, while examination showed a very large epithelial defect with an underlying Figure 2 Figure 3 ring infiltrate (Fig 1). At this point, the underlying history of soft contact lens use and long duration of the problem In the United Kingdom AK is more common than fungal keratitis raised the possibility of acanthaemoeba keratitis. Cultures and probably more common in contact lens wearers than HSV for acanthamoeba were negative, but confocal microscopy keratitis. AK should always be considered in contact lens patients showed the presence of numerous cysts in the corneal presenting with what appears to be HSV keratitis. -

Acanthamoeba Keratitis-A Diagnostic Dilemma: a Case Report
Advances in Ophthalmology & Visual System Case Report Open Access Acanthamoeba keratitis-a diagnostic dilemma: a case report Abstract Volume 8 Issue 1 - 2018 Acanthi amoeba keratitis presents as diagnostic dilemma as it mimics herpes simpex viral keratitis. It is also a diagnostic challenge due to lack of awareness, difficulty in Praveen Panwar, Kalpana Sharma Department of Ophthalmology, IGMC Shimla, India diagnosis which requires strong clinical suspicion and lack of effective treatment further complicates its management. We report a case of 47years old female with pain, redness Correspondence: Kalpana Sharma, Senior Resident Dept of and watering having deep stoma ring infiltrates. The strong suspicion based on clinical Ophthalmology, IGMC Shimla171001, India, Tel 9418004791, findings and failure to respond to antiviral therapy prompted us to investigate her further Email and the diagnosis of acanthi amoeba keratitis was made although there was no history of contact lens wear. Inspite of full diagnostic work up for bacterial, fungal and viral corneal Received: December 29, 2017 | Published: February 16, 2018 pathogen, the diagnosis of Acanthi amoeba can be easily missed, therefore one must keep the possibility of this rare entity. Keywords: acanthamoeba, viral keratitis, stoma ring infiltrates Introduction scrapings Acanthi amoeba cysts were observed (Figure 3).Bacterial, fungal as well culture on non nutrient agar enriched within E. coli Acanthi amoeba keratitis is a type of protozoa keratitis caused showed no growth. by free-living fresh water Amoeba. It recently gains importance because of increasing incidence, difficulty in diagnosis and unsatisfactory treatment. It is seen more commonly in contact lens wear using homemade saline or may be in non-contact lens wearers due to swimming or bathing in contaminated water.