CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

85EXT Bus Time Schedule & Line Route
85EXT bus time schedule & line map 85EXT West Enclave - Anand Vihar I.S.B.T View In Website Mode The 85EXT bus line (West Enclave - Anand Vihar I.S.B.T) has 8 routes. For regular weekdays, their operation hours are: (1) Anand Vihar I.S.B.T: 5:24 AM - 8:20 PM (2) Delhi Sectt.: 12:24 PM - 8:48 PM (3) Karam Pura T.: 12:28 PM (4) Punjabi Bagh: 2:40 PM (5) Sarswati Vihar W.Tank: 7:20 AM (6) Shivaji Stadium: 6:25 AM - 9:40 PM (7) Super Bazar: 2:36 PM (8) West Enclave: 7:26 AM - 10:35 PM Use the Moovit App to ƒnd the closest 85EXT bus station near you and ƒnd out when is the next 85EXT bus arriving. Direction: Anand Vihar I.S.B.T 85EXT bus Time Schedule 50 stops Anand Vihar I.S.B.T Route Timetable: VIEW LINE SCHEDULE Sunday 5:24 AM - 8:20 PM Monday 5:24 AM - 8:20 PM West Enclave Terminal Tuesday 5:24 AM - 8:20 PM Pitampura Depot Wednesday 5:24 AM - 8:20 PM Deepali Pitampura Thursday 5:24 AM - 8:20 PM Bhagwan Mahavir Hospital Friday 5:24 AM - 8:20 PM Keshav Mahavidhyalaya Saturday 5:24 AM - 8:20 PM Rani Bagh Rani Bagh 85EXT bus Info Direction: Anand Vihar I.S.B.T Raja Park Stops: 50 Trip Duration: 75 min Rajdhani Enclave / Mahindra Park Line Summary: West Enclave Terminal, Pitampura Depot, Deepali Pitampura, Bhagwan Mahavir Samrat Enclave Hospital, Keshav Mahavidhyalaya, Rani Bagh, Rani Bagh, Raja Park, Rajdhani Enclave / Mahindra Park, Shakur Pur Jj Colony/ Britannia Samrat Enclave, Shakur Pur Jj Colony/ Britannia, Shakurpur Village, Punjabi Bagh, East Punjabi Bagh, Shakurpur Village Punjabi Bagh Terminal, B Block New Moti Nagar, Karampura Terminal, -

313-LDS Bus Time Schedule & Line Route
313-LDS bus time schedule & line map 313-LDS Inderpuri - Delhi Secretariat (Ladies Sp) View In Website Mode The 313-LDS bus line (Inderpuri - Delhi Secretariat (Ladies Sp)) has 2 routes. For regular weekdays, their operation hours are: (1) Delhi Secretariat (Ladies Sp): 8:45 AM (2) Inderpuri (Ladies Sp): 6:05 PM Use the Moovit App to ƒnd the closest 313-LDS bus station near you and ƒnd out when is the next 313-LDS bus arriving. Direction: Delhi Secretariat (Ladies Sp) 313-LDS bus Time Schedule 30 stops Delhi Secretariat (Ladies Sp) Route Timetable: VIEW LINE SCHEDULE Sunday 8:45 AM Monday 8:45 AM Inderpuri Tuesday 8:45 AM Inder Puri (Krishi Kunj) Wednesday 8:45 AM Loha Mandi Thursday 8:45 AM Dev Prakash Shastri Road, New Delhi Friday 8:45 AM Bantax Saturday 8:45 AM Naraina Bus Depot Pandav Nagar Shadipur Dtc Colony Bus Stop 313-LDS bus Info Direction: Delhi Secretariat (Ladies Sp) Shadipur Metro Station Stops: 30 Trip Duration: 41 min Patel Road, Delhi Line Summary: Inderpuri, Inder Puri (Krishi Kunj), Shadipur Colony Loha Mandi, Bantax, Naraina Bus Depot, Pandav Nagar, Shadipur Dtc Colony Bus Stop, Shadipur West Patel Nagar Metro Station, Shadipur Colony, West Patel Nagar, Patel Nagar Metro Station, East Patel Nagar, Rajendra Place, Rajendra Place, Karol Bagh Metro Patel Nagar Metro Station Station, Karol Bagh Metro Station, Sadhu Vaswani Marg, Meghdoot Bhawan, Panchkuian Road East Patel Nagar Banvarilal Hospital, Panchkuian Road Banvarilal Hospital, Panchkuian Road, Sucheta Kriplani Rajendra Place Hospital, Super Bazar, Statesman House, Barakhamba Metro, Modern School, Mandi House, Rajendra Place Tilak Bridge, I.T.O., Delhi Secretariat Karol Bagh Metro Station Karol Bagh Metro Station Sadhu Vaswani Marg Meghdoot Bhawan Panchkuian Road Banvarilal Hospital Panchkuian Road Banvarilal Hospital Panchkuian Road Sucheta Kriplani Hospital Super Bazar Statesman House Barakhamba Metro Barakhamba Road, New Delhi Modern School Mandi House Tilak Bridge I.T.O. -

ML-33 Bus Time Schedule & Line Route
ML-33 bus time schedule & line map ML-33 Dwarka Sector-16 Metro Station - Karol Bagh View In Website Mode Metro Station The ML-33 bus line (Dwarka Sector-16 Metro Station - Karol Bagh Metro Station) has 2 routes. For regular weekdays, their operation hours are: (1) Dwarka Sector-16 Metro Station: 6:00 AM - 10:00 PM (2) Karol Bagh Metro Station: 6:00 AM - 10:00 PM Use the Moovit App to ƒnd the closest ML-33 bus station near you and ƒnd out when is the next ML-33 bus arriving. Direction: Dwarka Sector-16 Metro Station ML-33 bus Time Schedule 49 stops Dwarka Sector-16 Metro Station Route Timetable: VIEW LINE SCHEDULE Sunday 6:00 AM - 10:00 PM Monday 6:00 AM - 10:00 PM Karol Bagh Metro Station Tuesday 6:00 AM - 10:00 PM Karol Bagh (Telephone Exchange) Pusa Road, New Delhi Wednesday 6:00 AM - 10:00 PM Rajendra Place Thursday 6:00 AM - 10:00 PM Friday 6:00 AM - 10:00 PM East Patel Nagar Saturday 6:00 AM - 10:00 PM N.P.L. Colony R Block Rajendra Nagar Rajindar Nagar R-Block ML-33 bus Info Direction: Dwarka Sector-16 Metro Station Janak Vihar Stops: 49 Trip Duration: 59 min Line Summary: Karol Bagh Metro Station, Karol Pusa Institute Bagh (Telephone Exchange), Rajendra Place, East Patel Nagar, N.P.L. Colony, R Block Rajendra Nagar, Pusa Quarters Rajindar Nagar R-Block, Janak Vihar, Pusa Institute, Pusa Quarters, Nasc Dasghara, Todapur Village, Nasc Dasghara Monitoring And Receiving Station, Inderpuri A Block, Inderpuri, Inderpuri, Inder Puri (Krishi Kunj), Loha Todapur Village Mandi, Payal Cinema, Naraina Vihar, Mayapuri Depot, Government Press, -

807A Bus Time Schedule & Line Route
807A bus time schedule & line map 807A Old Delhi Railway Station (Fatehpuri) View In Website Mode The 807A bus line (Old Delhi Railway Station (Fatehpuri)) has 2 routes. For regular weekdays, their operation hours are: (1) Old Delhi Railway Station (Fatehpuri): 6:10 AM - 7:50 PM (2) Uttam Nagar Terminal: 6:50 AM - 9:30 PM Use the Moovit App to ƒnd the closest 807A bus station near you and ƒnd out when is the next 807A bus arriving. Direction: Old Delhi Railway Station (Fatehpuri) 807A bus Time Schedule 54 stops Old Delhi Railway Station (Fatehpuri) Route VIEW LINE SCHEDULE Timetable: Sunday 6:10 AM - 7:50 PM Uttam Nagar Terminal Monday 6:10 AM - 7:50 PM Uttam Nagar / A1 Janak Puri Tuesday 6:10 AM - 7:50 PM Tilak Pul Wednesday 6:10 AM - 7:50 PM Thursday 6:10 AM - 7:50 PM Jivan Park Friday 6:10 AM - 7:50 PM C-1 Janak Puri Saturday 6:10 AM - 7:50 PM A-3 Janak Puri C-2 Janakpuri 807A bus Info C2b Janak Puri Direction: Old Delhi Railway Station (Fatehpuri) Stops: 54 C4e Janakpuri Trip Duration: 78 min Line Summary: Uttam Nagar Terminal, Uttam Nagar C-3 Janakpuri / A1 Janak Puri, Tilak Pul, Jivan Park, C-1 Janak Puri, A-3 Janak Puri, C-2 Janakpuri, C2b Janak Puri, C4e C-4h Janakpuri Janakpuri, C-3 Janakpuri, C-4h Janakpuri, Janakpuri C-5a, Desu Colony, Sagarpur Vashisht Park, D Block Janakpuri C-5a Janak Puri, Lajwanti Garden, Zunk Market, Gulab House, Bobby Soap, Ps Mayapuri, Metal Forging, Desu Colony Government Press, Mayapuri Depot, Naraina Vihar / Indra Market, Payal Cinema, Loha Mandi, Bentax, Sagarpur Vashisht Park Naraina Depot, Pandav -

Study on Rajinder Nagar and Its Precincts
CITY LEVEL PROJECTS RAJINDER NAGAR Site Specific Design for Ward Number 149 Delhi Urban Art Commission The Delhi Urban Art Commission was set up by an Act of Parliament in 1973 to “advise the Government of India in the matter of preserving, developing and maintaining the aesthetic quality of urban and environmental design within Delhi and to provide advice and guidance to any local body in respect of any project of building operations or engineering operations or any development proposal which affects or is like to affect the skyline or the aesthetic quality of the surroundings or any public amenity provided therein”. II CITY LEVEL PROJect RAJINDER NAGAR 1 Delhi Urban Art Commission Prof. Dr. P.S.N. Rao Chairman Samir Mathur Member Abhimanyu Dalal Member Sonali Rastogi Member (till 02.07.2020) Kamran Rizvi Member & Addl. Secretary, Ministry of Housing and Urban Affairs (w.e.f 2.01.2020) DELHI URBAN ART COMMISSION with gratitude duly acknowledges the valuable contributions of the Ruby Kaushal Secretary (w.e.f 1.02.2019) following in making this report: Vinod Kumar Secretary (till 31.01.2019) Duac Staff Organisations / Others Ministry of Urban Development Rajeev Kumar Gaur, Amit Mukherji, Manju Anjali, Siddharth Sagar, Neha Chauhan Delhi Development Authority Government of National Capital Territory of Delhi North Delhi Municipal Corporation East Delhi Municipal Corporation South Delhi Municipal Corporation New Delhi Municipal Council Geospatial Delhi Limited Delhi Metro Rail Corporation Senior Consultant Delhi Urban Shelter Improvement Board Ameet Babbar BSES Rajdhani Power Limited Consultants BSES Yamuna Power Limited Parul Kapoor RWA’s and Area Councillors Mitali Kedia Google Earth 2 CITY LEVEL PROJect RAJINDER NAGAR 3 Preface *DISCLAIMER* This report is for academic purposes only and has been prepared on the basis of information gathered from various sources, in cases without any independent verification. -

1425553956.13Office-Order-List-Of-Hospitals-Delhi.Pdf
LIST OF EMPANELLED HOSPITALS/NURSING HOMES FOR DELHI & NCR Sl. Name of the Hospital No. 1 Medanta Medicity 1. Sector – 38, Gurgaon – 122001. Ph. No. 0124 441 1441 2 Metro Hospitals & Heart Institute X-1, Sector-11-12, L-94, Noida – 201301. Tel. No. 0120-252295 3 Kailash Hospital & Heart Institute H-33, Sector-27, Noida. Tel. No. 011- 2444444. 4 Asian Institute of Medical Sciences, Sec-21A, Badkal Flyover Road, Faridabad, Ph. No.0129-4253000. 5 Yashoda Hospital, III-M, Nehru Nagar, Ghaziabad. Ph. No. 0120- 418 2000 (30 lines), 0120- 2750001, 2.3.4 6 Dr. B.L. Kapur Memorial Hospital, 5 Pusa Road, New Delhi - 110005. Ph. No. 011 – 3040 3040. 7 Max Super Speciality Hospital (A Unit of Balaji Medical & Diagnostic Research Centre), 108-A, Indraprastha Extension, Patparganj, Delhi – 110092. Ph. No. 011 4303 3333. 8 Batra Hospital and Medical Research Centre, 1, Tughlakabad Institutional Area, Mehrauli Badarpur Road, New Delhi. Ph. No. 011- 29958747, 29957487, 29956431 9 Primus Super Specialty Hospital Chanakyapuri, New Delhi – 110021. Tel. No. 011-66206630, 66206640 10 National Heart Institute, 49-50, Community Centre, East of Kailash, New Delhi. Tel. No.011- 46606600. 11 Moolchand Hospital, Lajpat Nagar, III, New Delhi. Ph. No. 011 4200 0000. 12 Indian Spinal Injuries Centre Sector-C, Vasant Kunj, New Delhi – 110070. Tel. No. 011-42255225 13 Max Health Care, Block-B, Shushant Lok, Phase-I, Gurgaon-01. Tel.095124-6623000 14 Jeewan Nursing Home & Hospital,2-B, Pusa Road, Karol Bagh, New Delhi – 110005. Tel. No. 011-42430246. 15 Yashoda hospital Kaushambi, Ghaziabad Tel. -

871A Bus Time Schedule & Line Route
871A bus time schedule & line map 871A Shivaji Stadium Terminal View In Website Mode The 871A bus line (Shivaji Stadium Terminal) has 2 routes. For regular weekdays, their operation hours are: (1) Shivaji Stadium Terminal: 11:00 AM - 9:50 PM (2) Uttam Nagar Terminal: 9:50 AM - 9:30 PM Use the Moovit App to ƒnd the closest 871A bus station near you and ƒnd out when is the next 871A bus arriving. Direction: Shivaji Stadium Terminal 871A bus Time Schedule 40 stops Shivaji Stadium Terminal Route Timetable: VIEW LINE SCHEDULE Sunday 11:00 AM - 9:50 PM Monday 11:00 AM - 9:50 PM Uttam Nagar Terminal Tuesday 11:00 AM - 9:50 PM Vikas Puri Crossing Najafgarh Road, New Delhi Wednesday 11:00 AM - 9:50 PM Dholi Piao Thursday 11:00 AM - 9:50 PM Friday 11:00 AM - 9:50 PM District Centre Najafgarh Road Saturday 11:00 AM - 9:50 PM Janakpuri East Metro Station/ Nangli Zalib Check Post Janak Puri Tilak Nagar Crossing (Najafgarh Road) 871A bus Info Direction: Shivaji Stadium Terminal Sant Pura Tilak Nagar Stops: 40 Trip Duration: 47 min Meenakshi Garden Line Summary: Uttam Nagar Terminal, Vikas Puri Crossing, Dholi Piao, District Centre Najafgarh Road, Janakpuri East Metro Station/ Nangli Zalib, Check Subhash Nagar Crossing / Mukherji Park Post Janak Puri, Tilak Nagar Crossing (Najafgarh Road), Sant Pura Tilak Nagar, Meenakshi Garden, Tagore Garden Subhash Nagar Crossing / Mukherji Park, Tagore Garden, Tagore Garden (Gurudwara Road), Rajouri Tagore Garden (Gurudwara Road) Garden, Raja Garden, Bali Nagar, Basai Darapur / Ramesh Nagar, Kirti Nagar, Moti Nagar -

522-LDS Bus Time Schedule & Line Route
522-LDS bus time schedule & line map 522-LDS Ambedkar Nagar Terminal - Rajendra Place View In Website Mode (Ladies Sp) The 522-LDS bus line (Ambedkar Nagar Terminal - Rajendra Place (Ladies Sp)) has 2 routes. For regular weekdays, their operation hours are: (1) Ambedkar Nagar Terminal (Ladies Sp): 6:00 PM (2) Rajendra Place (Ladies Sp): 9:00 AM Use the Moovit App to ƒnd the closest 522-LDS bus station near you and ƒnd out when is the next 522-LDS bus arriving. Direction: Ambedkar Nagar Terminal (Ladies Sp) 522-LDS bus Time Schedule 50 stops Ambedkar Nagar Terminal (Ladies Sp) Route VIEW LINE SCHEDULE Timetable: Sunday 6:00 PM Rajendra Place Monday 6:00 PM Rajendra Place Tuesday 6:00 PM Karol Bagh Metro Station Wednesday 6:00 PM Thursday 6:00 PM Karol Bagh Metro Station Friday 6:00 PM Sadhu Vaswani Marg Saturday 6:00 PM Meghdoot Bhawan Panchkuian Road Banvarilal Hospital 522-LDS bus Info Balmiki Mandir Direction: Ambedkar Nagar Terminal (Ladies Sp) Stops: 50 Police Station Mandir Marg Trip Duration: 65 min Line Summary: Rajendra Place, Rajendra Place, Gole Market Karol Bagh Metro Station, Karol Bagh Metro Station, Sadhu Vaswani Marg, Meghdoot Bhawan, Gole Market Panchkuian Road Banvarilal Hospital, Balmiki Mandir, Police Station Mandir Marg, Gole Market, Rama Krishna Ashram Marg Gole Market, Rama Krishna Ashram Marg, Rama Krishna Ashram Marg, Panchkuian Road, Sucheta Rama Krishna Ashram Marg Kriplani Hospital, Super Bazar, Kailash Building, Tolstoy Marg, Eastern Court, Windsor Place, National Panchkuian Road Archives, National Museum, -

CONTACT LIST CGHS DELHI - WELLNESS CENTRES CGHS EAST ZONE Allopathic Wcs CGHS Under East Zone S Name of Address Contact Number Official E-Mail ID No
CONTACT LIST CGHS DELHI - WELLNESS CENTRES CGHS EAST ZONE Allopathic WCs CGHS under East Zone S Name of Address Contact Number Official e-mail ID No. Wellness Centre and Code 1 Chandni Chowk Near Moti Cinema, Chandni Chowk, Delhi-6 011-23866892 [email protected] D8 2 Darya Ganj D28 Sir Sayed Ahmed Road, Post Office Wali Gali, Behind 011-23273043 [email protected] Golcha Cinema Delhi -110002 3 Dilshad Garden Pocket-B, Near Sarvodaya Vidyalaya, Dilshad Garden, 011-22596489 [email protected] D87 Delhi - 110095 4 Ghaziabad D68 CGHS Building, Near Kendriya Vidyalaya, Kamla Nehru 0120-2789969 [email protected] Nagar, Ghaziabad, UP-201002 5 GKG WC D56 F3/23,Krishna Nagar(Near Vijay Chowk),Delhi-51 011-22512866 [email protected] 6 Greater Noida P3 Shopping complex, First floor, Greater Noida-201310 0120-2447126 [email protected] D22A 7 Kingsway Camp CGHS Wellness Centre, New Police Line, Kingsway 011-27459411 [email protected] D60 Camp, Delhi 8 Laxminagar D67 CGHS Building, H-Block, Vikas Marg (Near Metro 011-22543108/ 011- [email protected] Station),Laxmi Nagar, Delhi-110092 22466544 9 Mayur Vihar D77 264 A, Mayur Vihar, Pocket-I, Phase-I, Trilok Kunj , 011-22751321 [email protected] Delhi-110091 10 Noida Sec-82 Community Center I, Kendriya Vihar II, Plot No. 3, 0120-2568899 [email protected] D95 Sector 82, Noida-201304 11 Noida D85 R 51, Near Modern School, Sector-11, NOIDA, UP- 0120-2532312 [email protected] 201301. -

New Delhi Office Name of the Regional Office : New Delhi S No Name & Regd
(Updated as on September 30, 2021) (a) List containing names and addresses of FFMCs licenced by the New Delhi Office Name of the Regional Office : New Delhi S No Name & regd. address of the FFMC Address of the Branch 11Century Travels Pvt Ltd. G-39, 2nd Floor, Pawan House G-39, 2nd Floor, Pawan House Connaught Place, New Delhi – 110001 Connaught Place, New Delhi – 110001 21BNA Enterprises Pvt Ltd. 5 Ansari Road, Daryaganj, 5 Ansari Road, Daryaganj, New Delhi – 110002 New Delhi – 110002 31Sultanjee Enterprises Pvt Ltd. 3633, Park Mansion, (Above Post Office) 3633, Park Mansion, (Above Post Office) Darya Ganj, New Delhi – 110002 Darya Ganj, New Delhi – 110002 41New Airways Travels (Delhi) Pvt. Ltd. 84, Tolstoy Lane, 84, Tolstoy Lane, Janpath, New Delhi – 110001 Janpath, New Delhi – 110001 51Sky Link Travel Pvt. Ltd. G-17, Marine Arcade, G-17, Marine Arcade, Connaught Place, New Delhi – 110001 Connaught Place, New Delhi – 110001 61G.D. Goenka Tourism Corporation Ltd. N-86, Connaught Place, N-86, Connaught Place, New Delhi – 110001 New Delhi – 110001 71Aadi Satya Travels Pvt. Ltd. 24-B/4, DB Gupta Road 24-B/4, DB Gupta Road Dev Nagar, Karol Bagh, New Delhi – 110005 Dev Nagar, Karol Bagh, New Delhi – 110005 81Sethi Bros Pvt Ltd. 16/10159, Padam SinghRoad, Naiwala,Opp. Shastri 16/10159, Padam Singh Road, Naiwala,Opp. Shastri Market Parking, Karol Bagh, New Delhi- 110005 Market Parking, Karol Bagh, New Delhi- 110005 91New Foundland Enterprises Pvt. Ltd. Shop No. 46, Municipal Market, Outer Circle, GF-12, Indraprakash Building Connaught Place, New Delhi 110 001 21, Barakhamba Road, New Delhi – 110001 10CDS Money Exchange Bureau Pvt. -

Government Testing Centres in New Delhi for COVID – 19
Government testing centres in New Delhi for COVID – 19 All India Institute Medical Sciences Sri Aurobindo Marg, Ansari Nagar, Ansari Nagar East, New Delhi, Delhi 110029 Lady Hardinge Medical College Connaught Place, New Delhi, Delhi 110001 National Centre for Disease Control 22, Sham Nath Marg, Civil Lines, New Delhi, Delhi 110054 Ram Manohar Lohia Hospital Baba Kharak Singh Rd, near Gurudwara Bangla Sahib, Ram Manohar Lohia Hospital, Type III, Connaught Place, New Delhi, Delhi 110001 Institute of Liver & Biliary Sciences D1 ILBS Road, Vasant Kunj, New Delhi, Delhi 110070 Army Hospital Research & Referral Near, Military Hospital Road, Subroto Park, Dhaula Kuan, New Delhi, Delhi 110010 Delhi Maulana Azad Medical College 2, Bahadur Shah Zafar Marg, Maulana Azad Medical College Campus, Balmiki Basti, New Delhi, Delhi 110002 Government approved testing centres in New Delhi for COVID – 19 Government approved testing Centres Central District Hotel Apra Inn, 15A/61 WEA Colony, Ajmal Khan Road, Karol Bagh East District Swami Parmanand Prakritik Chikitsalaya Yoga & Anushandhan Kendra, West Vinod Nagar North District Shrine Hospital, Main Bawana Road, Sector 17, Rohini (connected with MVH Hospital) North West District Maharaja Agrasen Bhawan CS Pocket 12, Rithala Road, Sector 5, Rohini Khosla Hospital, Shalimar Bagh Shri Satnam Bhawan, Block C3, Phase 2, Ashok Vihar, Delhi Quarantine Facility, DUSIB Flats, ES Block, Sultan Puri Prime Stay B & B, Haiderpur, Shalimar Bagh Shahdara District Hotel Park, Shahdara South District Beleamonde Hotel, Chhatarpur, -
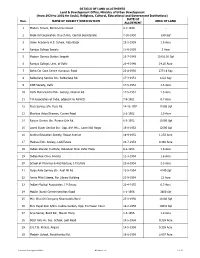
Allotment List 1 of 27 DATE of Sno
DETAILS OF LAND ALLOTMENTS Land & Development Office, Ministry of Urban Development (from 1930 to 2002 for Social, Religious, Cultural, Educational and Government Institutions) DATE OF Sno. NAME OF SOCIETY/INSTITUTION AREA OF LAND ALLOTMENT 1 Modern School, Barakhamba Road 4-7-1930 - 2 Prem Oil Corporation Church Rd., Central Secretariate 7-10-1930 240 Sqf 3 Union Academy H.S. School, Raja Bazar 13-2-1939 1.6 Acre 4 Ramjas College Society 17-6-1939 3 Acre 5 Modern Service Station Janpath 23-7-1943 11803.50 Sqf 6 Ramjas College, Univ. of Delhi 29-4-1946 14.25 Acre 7 Batra Car Care Centre Humayun Road 23-8-1950 2371.6 Sqy 8 Safdarjang Service Stn. Safdarjang Rd. 17-3-1951 1222 Sqy 9 DME Society, Delhi 17-5-1951 1.5 Acre 10 Delhi Maharashtra Edn. Society, Original Rd. 17-5-1951 1.5 Acre 11 T.B.Association of India, adjacent to AIFACS 7-9-1951 0.7 Acre 12 Pusa Service Stn. Pusa Rd. 14-12-1951 11000 Sqf 13 Bhartiya Vidya Bhawan, Curzen Road 2-2-1952 1.9 Acre 14 Rajeev Service Stn. Purana Qila Rd. 6-3-1952 11000 Sqf 15 Laxmi Super Service Stn. Opp. INA Mkt., Laxmi Bai Nagar 19-8-1952 12000 Sqf 16 Andhra Education Society, Rouse Avenue 18-9-1952 1.233 Acre 17 Madrasi Edn. Society, Lodi Estate 23-7-1953 0.386 Acre 18 Indian Standar Institute, Bahaduar Shah Zafar Marg 9-2-1954 1.6 Acre 19 Indian Red Cross Society 11-3-1954 1.8 Acre 20 School of Planning & Architecture, I.P.Estate 23-8-1954 2.5 Acre 21 Paras Auto Service Stn.