Cbc Storeroom Chemical Price Listing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Calcium Nitrate Calcium Nitrate Is a Highly Soluble Source of Two Plant Nutrients
No. 27 Calcium Nitrate Calcium nitrate is a highly soluble source of two plant nutrients. Its high solubility makes it popular for supplying an immediately available source of nitrate and calcium directly to soil, through irrigation water, or with foliar applications. Production: Phosphate rock is acidified with nitric acid to form a mixture of phosphoric acid and calcium nitrate during the nitrophosphate fertilizer manufacturing process. Ammonia is then added to neutralize excess acidity. Calcium nitrate crystals precipitate via a temperature gradient and are separated as the mixture is cooled. With the ammonia addition and crystallization, a double salt is formed [5 Ca(NO3)2•NH4NO3•10 H2O, referred to as 5:1:10 double salt] and is considered the commercial grade of calcium nitrate. Hence, small amounts of ammonical N may also be present in this grade of calcium nitrate. Calcium nitrate is also manufactured by reacting nitric acid with crushed limestone forming either the 5:1:10 double salt or calcium nitrate tetrahydrate (Ca(NO3)2•4 H2O). The latter product is often produced as a wet crystal or a mesh and is subject to specific regulation with respect to handling and safety. Prilling and granulating are the most common methods of making particles ready for field use. Calcium nitrate is very hygroscopic (absorbs water from the air), so when intended for soil application, proprietary coatings are applied to minimize moisture uptake. Calcium nitrate intended for hydroponics or fertigation does not contain a conditioner, or it may be sold -

Effects of Different Sources of Fertilizer Nitrogen on Growth and Nutrition of Western Hemlock Seedlings
Effects of Different Sources U.S. Department of Agriculture Forest Service Pacific Northwest Forest of FertiIizer Nitrogen and Range Experiment Station Research Paper PNW-267 on Growth and Nutrition oJ February 1980 Western Hemlock Seedlings ---. --_. ------------------------ , I _J Authors M. A. RADWAN is Principal Plant Physiologist and DEAN S. DeBELL is Principal Silviculturist with the Forest Service, u.S. Department of Agriculture, Pacific Northwest Forest and Range Experiment Station, Forestry Sciences Laboratory, Olympia, Washington. En gl ish Equivalents 1 liter 0.2642 gallon 1 kilogram = 2.2046 pound 1 gram = 0.0353 ounce 1 centimeter = 0.3937 inch 1 kilogram per hectare 1.1206 pounds per acre (9/50C) + 32 = of EFFECTS OF DIFFERENT SOURCES OF FERTILIZER NITROGEN ON GROWTH AND NUTRITION OF WESTERN HEMLOCK Reference Abstract Radwan, M. A. , and Dean S. DeBell. 1980. Effects of different sources of fertilizer nitrogen on growth and nutrition of western hemlock seedlings. USDA For. Servo Res. Pap. PNW-267, 15 p. Pacific Northwest Forest and Range Experiment Station, Portland, Oregon. Twelve different nitrogen (N) fertilizer treatments were tested on potted western hemlock (Tsuga heterophylla (Raf. ) Sarg.) seedlings. Fertilizers affected soil N and pH, and growth and foliar chemical com position of seedlings. Ura plus N-Serve and sulfur-coated urea appear more promising for promoting growth than other fertilizers tested. Results, however, do not explain reported variability in response of hemlock stands to N fertilization. Keywords: Nitrogen fertilizer response, seedling growth, western hemlock, Tsuga heterophylla. RESEARCH SUMMARY Research Paper PNW-267 1980 The following fertilization treatments were applied in the spring to potted, 4-year-old western hemlock (Tsuga heterophylla (Raf. -

CAN-17 Calcium Ammonium Nitrate 17-0-0 GUARANTEED ANALYSIS TOTAL NITROGEN (N)
Product Data Sheet CAN-17 Calcium Ammonium Nitrate 17-0-0 GUARANTEED ANALYSIS TOTAL NITROGEN (N) ...................................................................................................................................17.00% 5.40% Ammoniacal Nitrogen 11.60% Nitrate Nitrogen Calcium (Ca) ......................................................................................................................................................8.80% Derived from Ammonium Nitrate and Calcium Nitrate. KEEP OUT OF REACH OF CHILDREN 3. This product is a commercial fertilizer used as plant WARNING food in agricultural crop production. For specific CAUSES SKIN IRRITATION. application rates follow the recommendation of a CAUSES SERIOUS EYE IRRITATION. qualified individual or institution, such as, but not PRECAUTIONARY STATEMENTS: Wash thoroughly after limited to, a certified crop advisor, agronomist, handling. Specific treatment see First Aid section on this label. university crop extension publication, or apply PERSONAL PROTECTIVE EQUIPMENT: Wear protective according to recommendations in your approved gloves / protective clothing / eye protection / face protection. Take off nutrient management plan. contaminated clothing and wash before reuse. FIRST AID: IF ON SKIN Wash with plenty of water. If skin ir- ADVANTAGES ritation occurs, get medical advice / attention. IF IN EYES Rinse cautiously with water for several minutes. Remove contact lenses, if 1. Contains two forms of nitrogen– Nitrate nitrogen, fast present and easy to do. Continue rinsing. -

Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses
Journal of Functional Biomaterials Article Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses Alejandra Ruiz-Clavijo 1,2, Andrew P. Hurt 2, Arun K. Kotha 2 and Nichola J. Coleman 2,* 1 Facultad de Ciencias Químicas, Universidad Complutense de Madrid, Av. Complutense, 28040 Madrid, Spain; [email protected] 2 Faculty of Engineering and Science, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK; [email protected] (A.P.H.); [email protected] (A.K.K.) * Correspondence: [email protected]; Tel.: +44-208-331-9825 Received: 3 January 2019; Accepted: 20 February 2019; Published: 23 February 2019 Abstract: This study investigated the impact of different calcium reagents on the morphology, composition, bioactivity and biocompatibility of two-component (CaO-SiO2) glasses produced by the Stöber process with respect to their potential application in guided tissue regeneration (GTR) membranes for periodontal repair. The properties of the binary glasses were compared with those of pure silica Stöber particles. The direct addition of calcium chloride (CC), calcium nitrate (CN), calcium methoxide (CM) or calcium ethoxide (CE) at 5 mol % with respect to tetraethyl orthosilicate in the reagent mixture gave rise to textured, micron-sized aggregates rather than monodispersed ~500 nm spheres obtained from the pure silica Stöber synthesis. The broadening of the Si-O-Si band at ~1100 cm−1 in the infrared spectra of the calcium-doped glasses indicated that the silicate network was depolymerised by the incorporation of Ca2+ ions and energy dispersive X-ray analysis revealed that, in all cases, the Ca:Si ratios were significantly lower than the nominal value of 0.05. -

Methanogenesis Rates in Acetate and Nitrate Amended Anoxic Slurries
Methanogenesis rates in acetate and nitrate amended anoxic slurries BIOS 35502: Practicum in Environmental Field Biology Patrick Revord Advisor: William West 2011 Abstract With increasing urbanization and land use changes, pollution of lakes and wetland ecosystems is imminent. Any influx of nutrients, anthropogenic or natural, can have dramatic effects on lake gas production and flux. However, the net effect of simultaneous increase of both acetate and nitrate is unknown. Methane (CH4) production was measured in anoxic sediment and water slurries amended with ammonium nitrate (NH4NO3), which has been shown to inhibit methanogenesis, and sodium acetate (CH3COONa or NaOAc), which is known to increase methanogenesis. The addition of acetate significantly increased the methanogenesis rate, but the nitrate amendment had no significant effect. The simultaneous amendment of both acetate and nitrate showed no significant increase in CH4 compared to the control, indicating that the presence of nitrate may have reduced the effect of acetate amendment. Introduction Methane, a greenhouse gas associated with global warming, continues to increase in concentration in our atmosphere. Global yearly flux of methane into the atmosphere is 566 teragrams of CH4 per year, which is more than double pre-industrial yearly flux (Solomon et al. 2007). Increasing urbanization and land-use changes contribute significantly to increased gas levels (Anderson et al. 2010, Vitousek 1994). Nutrients travel from anthropogenic sources such as wastewater treatment facilities, landfills, and agricultural plots into nearby lakes, rivers, and wetlands, causing increased primary productivity in a process known as eutrophication (Vitousek et al. 1997). The increased nutrients and productivity lead to toxic algal blooms that create products such as acetate, H2, and CO2; a nutrient-rich anoxic environment suitable for anaerobic bacteria to produce unnaturally high levels of methane and other greenhouse gases (Davis and Koop 2006, West unpublished data). -

Customer Information Regarding Material Resistance in Compressed Air Preparation
Customer information regarding material resistance in compressed air preparation Polycarbonate reservoirs for filter regulators, filters, and lubricators Everywhere the presence of these media cannot be avoided, such as in paint booths, gluing machines, Polycarbonate is the longest known and most vulcanization plants, etc., the use of metal reservoirs processed material in the world for reservoirs of is required. compressed air maintenance units. The high Problematic is the use of solvents not only in pressure and temperature resistances, as well as immediate contact with the reservoir, but also in its good compatibility with the normally used media immediate vicinity. For example, trichlorethylene such as water, oils and greases are some of its vapors from the compressor’s intake air can cause advantages. crack formation in the polycarbonate reservoir. Only The only “weakness” of this plastic is its clean the reservoirs using a slightly damp cloth. Only susceptibility to media that can be referred to use water to do this and, if necessary, a mild collectively using the term “solvents”. From this detergent without chemical additives. range, the materials relevant to the use of If a lubricator is used, please only use suitable compressed air preparation units are summarized pneumatic oils, e.g. AVENTICS pneumatic oil, order here: no. 8982000010 – 1L. Detergents : Trichloroethylene, (usually from outside) perchloroethylene, In most cases, the use of (pneumatic) oils with benzene, additives, for example antifreeze, results in damage super/regular gasoline to or destruction of the reservoirs and must thus be avoided. Alternatively, we recommend the use of Solvents : Acetone, metal reservoirs. (from outside/inside) paint thinners, alcohols, esters Polycarbonate can react to permanent UV-light irradiation and weathering. -

NMR Chemical Shifts of Common Laboratory Solvents As Trace Impurities
7512 J. Org. Chem. 1997, 62, 7512-7515 NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E. Gottlieb,* Vadim Kotlyar, and Abraham Nudelman* Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel Received June 27, 1997 In the course of the routine use of NMR as an aid for organic chemistry, a day-to-day problem is the identifica- tion of signals deriving from common contaminants (water, solvents, stabilizers, oils) in less-than-analyti- cally-pure samples. This data may be available in the literature, but the time involved in searching for it may be considerable. Another issue is the concentration dependence of chemical shifts (especially 1H); results obtained two or three decades ago usually refer to much Figure 1. Chemical shift of HDO as a function of tempera- more concentrated samples, and run at lower magnetic ture. fields, than today’s practice. 1 13 We therefore decided to collect H and C chemical dependent (vide infra). Also, any potential hydrogen- shifts of what are, in our experience, the most popular bond acceptor will tend to shift the water signal down- “extra peaks” in a variety of commonly used NMR field; this is particularly true for nonpolar solvents. In solvents, in the hope that this will be of assistance to contrast, in e.g. DMSO the water is already strongly the practicing chemist. hydrogen-bonded to the solvent, and solutes have only a negligible effect on its chemical shift. This is also true Experimental Section for D2O; the chemical shift of the residual HDO is very NMR spectra were taken in a Bruker DPX-300 instrument temperature-dependent (vide infra) but, maybe counter- (300.1 and 75.5 MHz for 1H and 13C, respectively). -
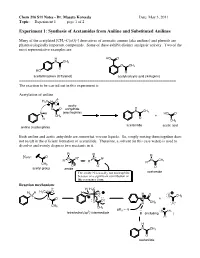
Experiment 1: Synthesis of Acetamides from Aniline and Substituted Anilines
Chem 216 S11 Notes - Dr. Masato Koreeda Date: May 3, 2011 Topic: __Experiment 1____ page 1 of 2. Experiment 1: Synthesis of Acetamides from Aniline and Substituted Anilines Many of the acetylated [CH3–C(=O)-] derivatives of aromatic amines (aka anilines) and phenols are pharmacologically important compounds. Some of these exhibit distinct analgesic activity. Two of the most representative examples are: H HO O N CH3 O CH3 O HO O acetaminophen (Tylenol) acetylsalicylic acid (Aspirin) ======================================================================= The reaction to be carried out in this experiment is: Acetylation of aniline δ- H3C δ+ O acetic O O anhydride H N CH3 N H (electrophile) HO O CH3 + H O CH3 acetanilide acetic acid aniline (nucleophile) Both aniline and acetic anhydride are somewhat viscous liquids. So, simply mixing them together does not result in the efficient formation of acetanilide. Therefore, a solvent (in this case water) is used to dissolve and evenly disperse two reactants in it. R R R Note: O + N R" N R" N CH R' R' R' 3 CH3 - O O O acetyl group amide The amide N is usually not nucleophilic acetamide because of a significant contribution of this resonance form. Reaction mechanism: δ- H H3C H H H3C δ+ O H N O H O N O CH3 O O N + O O H CH3 O CH3 CH3 pKa ~ -5 3 O CH3 tetrahedral (sp ) intermediate B (including ) O H N CH3 O acetanilide Chem 216 S11 Notes - Dr. Masato Koreeda Date: May 3, 2011 Topic: __Experiment 1____ page 2 of 2. Additional comments on the reaction mechanism: 1. -

The Chiba System 千葉方式 : a Non Toxic Alternative to the Dichromate
The Chiba System 千葉方式 A Non Toxic Alternative to the Dichromate Processes January 2007 Halvor Bjoerngaard Graduate School of Science and Technology CHIBA UNIVERSITY (千葉大学学位申請論文) The Chiba System 千葉方式 : A Non Toxic Alternative to the Dichromate Processes or The Production of Photographic Prints in Permanent Pigments by Utilising the Sensitivity of the Ferric Salt to the Spectre and Employing the Polymerization of Colloids. 2007年1月 千葉大学大学院自然科学研究科 情報科学専攻画像科学 Halvor Bjørngård Abstract This study has the main purpose of presenting a non-toxic, or an alternative, printing system for the dichromate based pigment processes. The two methods presented in depth are modelled on first Carbon printing then Gum Printing. Achieving non-toxicity for these systems means replacing the dichromate sensitizer and secondly to avoid the practise of hardening the substrate. An alternative sensitizer is presented and hardening is avoided by using modified working methods. The chemistry utilised for this purpose is iron based, red-ox induced, free radical polymerization. The sensitizer is ammonium ferric citrate, using either hydrogen peroxide or ammonium persulphate as developer. For Carbon Printing a solution to both the need for hardeners and the problem of oxygen inhibition, which is usual for this kind of polymerisation, is achieved. This is done by using a covering layer of agar-agar that blocks oxygen and changes the transfer system, obsolescing the use of hardeners. For Gum Printing two methods are presented. One is based on gelatine, which allows the use of a hydrogen peroxide bath for development. The second method is with gum arabicum, which necessitates inclusion of ammonium persulphate in the coating as a developing agent. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials
materials Article Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials Akira Yoneyama 1, Heesup Choi 1,* , Masumi Inoue 1, Jihoon Kim 2, Myungkwan Lim 3,* and Yuhji Sudoh 4 1 Department of Civil and Environmental Engineering, Kitami Institute of Technology, Hokkaido 090-8507, Japan; [email protected] (A.Y.); [email protected] (M.I.) 2 Faculty of Environmental Technology, Muroran Institute of Technology, Hokkaido 090-8585, Japan; [email protected] 3 Department of Architectural Engineering, Songwon University, Gwangju 61756, Korea 4 Basic Chemicals Department Chemicals Division, Nissan Chemical Corporation, Tokyo 103-6119, Japan; [email protected] * Correspondence: [email protected] (H.C.); [email protected] (M.L.) Abstract: Recently, there has been increased use of calcium-nitrite and calcium-nitrate as the main components of chloride- and alkali-free anti-freezing agents to promote concrete hydration in cold weather concreting. As the amount of nitrite/nitrate-based accelerators increases, the hydration of tricalcium aluminate (C3A phase) and tricalcium silicate (C3S phase) in cement is accelerated, thereby improving the early strength of cement and effectively preventing initial frost damage. Nitrite/nitrate-based accelerators are used in larger amounts than usual in low temperature areas ◦ below −10 C. However, the correlation between the hydration process and strength development in concrete containing considerable nitrite/nitrate-based accelerators remains to be clearly identified. Citation: Yoneyama, A.; Choi, H.; In this study, the hydrate composition (via X-ray diffraction and nuclear magnetic resonance), pore Inoue, M.; Kim, J.; Lim, M.; Sudoh, Y. -

Sodium Acetate
SODIUM ACETATE Prepared at the 18th JECFA (1974), published in NMRS 54B (1975) and in FNP 52 (1992). Metals and arsenic specifications revised at the 59th JECFA (2002). An ADI not limited' was established at the 17th JECFA (1973) SYNONYMS INS No. 262(i) DEFINITION Chemical names Sodium acetate C.A.S. number 127-09-3 Chemical formula C2H3NaO2 · nH2O (n = 0 or 3) Structural formula CH3COONa · nH2O (n = 0 or 3) Formula weight Anhydrous: 82.03 Trihydrate: 136.08 Assay Not less than 98.5% after drying DESCRIPTION Anhydrous: White, odourless, granular, hygroscopic powder Trihydrate: Colourless, transparent crystals or a granular crystalline powder, odourless or with a faint, acetic odour. Effloresces in warm, dry air. FUNCTIONAL USES Buffer CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Very soluble in water; soluble in ethanol pH (Vol. 4) 8.0 - 9.5 (1 in 100 soln) Test for sodium (Vol. 4) Passes test Test for acetate (Vol. 4) Passes test Heat test Anhydrous: When heating the sample slowly, it first fuses gradually and boils, and later decomposes evolving an unpleasant odour of acetone. A solution of the residue gives alkaline reaction with litmus paper. Trihydrate: When heating the sample slowly, it liquefies. Then water evaporates, and a powder forms. By heating more strongly, the powder fuses, and becomes lumpy and later decomposes evolving an odour of acetone. A solution of the residue gives alkaline reaction with litmus paper. PURITY Loss on drying (Vol. 4) Anhydrous: Not more than 2.0% (120o, 4 h) Trihydrate: Between 36 and 42% (120o, 4 h) Test for potassium Negative test (Vol.