COVID-19 Anosmia Reporting Tool: Initial Findings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Second Edition
COVID-19 Evidence Update COVID-19 Update from SAHMRI, Health Translation SA and the Commission on Excellence and Innovation in Health Updated 4 May 2020 – 2nd Edition “What is the prevalence, positive predictive value, negative predictive value, sensitivity and specificity of anosmia in the diagnosis of COVID-19?” Executive Summary There is widespread reporting of a potential link between anosmia (loss of smell) and ageusia (loss of taste) and SARS-COV-2 infection, as an early sign and with sudden onset predominantly without nasal obstruction. There are calls for anosmia and ageusia to be recognised as symptoms for COVID-19. Since the 1st edition of this briefing (25 March 2020), there has been a significant expansion of literature on this topic, including 3 systematic reviews. Predictive value: The reported prevalence of anosmia/hyposmia and ageusia/hypogeusia in SARS-COV-2 positive patients are in the order of 36-68% and 33-71% respectively. There are reports of anosmia as the first symptom in some patients. Estimates from one study for hyposmia and hypogeusia: • Positive likelihood ratios: 4.5 and 5.8 • Sensitivity: 46% and 62% • Specificity: 90% and 89% The US Centres for Disease Control and Prevention (CDC) has added new loss or taste or smell to its list of recognised symptoms for SARS-COV-2 infection. To date, the World Health Organization has not. Conclusion: There is sufficient evidence to warrant adding loss of taste and smell to the list of symptoms for COVID-19 and promoting this information to the public. Context • Early detection of COVID-19 is key to the ongoing management of the pandemic. -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Clinical Diagnosis and Treatment of Olfactory Dysfunction
Clinical Diagnosis and Treatment of Olfactory Dysfunction Seok Hyun Cho Hanyang Med Rev 2014;34:107-115 http://dx.doi.org/10.7599/hmr.2014.34.3.107 Department of Otorhinolaryngology-Head and Neck Surgery, Hanyang University College of Medicine, Seoul, Korea pISSN 1738-429X eISSN 2234-4446 Olfactory dysfunction is a relatively common disorder that is often under-recognized by Correspondence to: Seok Hyun Cho Department of Otorhinolaryngology-Head both patients and clinicians. It occurs more frequently in older ages and men, and decreases and Neck Surgery, Hanyang University patients’ quality of life, as olfactory dysfunction may affect the emotion and memory func- Hospital, 222 Wangsimni-ro, Seongdong-gu, tions. Three main causes of olfactory dysfunction are sinonasal diseases, upper respiratory Seoul 133-792, Korea Tel: +82-2-2290-8583 viral infection, and head trauma. Olfactory dysfunction is classified quantitatively (hypos- Fax: +82-2-2293-3335 mia and anosmia) and qualitatively (parosmia and phantosmia). From a pathophysiologi- E-mail: [email protected] cal perspective, olfactory dysfunction is also classified by conductive or sensorineural types. All patients with olfactory dysfunction will need a complete history and physical examina- Received 17 April 2014 Revised 23 June 2014 tion to identify any possible or underlying causes and psychophysical olfactory tests are Accepted 3 July 2014 essential to estimate the residual olfactory function, which is the most important prognos- This is an Open Access article distributed under tic factor. CT or MRI may be adjunctively used in some indicated cases such as head trauma the terms of the Creative Commons Attribution and neurodegenerative disorders. -

Both Anxiety and Joint Laxity Determine the Olfactory Features in Panic Disorder
Psychiatry Research xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Psychiatry Research journal homepage: www.elsevier.com/locate/psychres Both anxiety and joint laxity determine the olfactory features in panic disorder ⁎ Emma Buróna, , Antonio Bulbenaa,b, Andrea Bulbena-Cabréa,c, Sílvia Rosadob, Guillem Pailhezb a Autonomous University of Barcelona, Department of Psychiatry and Forensic Medicine (UAB), Bellaterra, Spain b Mar Health Park, Neuropsychiatry and Drug Addiction Institute (INAD), Barcelona, Spain c Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA ARTICLE INFO ABSTRACT Keywords: Previous research showed a high sensitivity in sensorial modalities in panic disorder (PD). This disorder has been Panic disorder consistently associated to the joint hypermobility syndrome (JHS). In non-clinical samples, this collagen al- Olfactory threshold teration has been also related to an enhanced sensitivity in some sensorial modalities. The main aim of this study Smell reactivity is to explore the olfactory functioning in PD in relation to JHS. Sixty patients with PD and sixty healthy controls Olfactory awareness performed the Sniffin’ Sticks Test (SST) (threshold subtest), and completed the Affective Impact of Odors scale Joint hypermobility syndrome (AIO), the Relational Scale of Olfaction (EROL), and the Odor Awareness Scale (OAS). Clinical symptom rating scales and JHS assessment were also obtained. PD patients showed enhanced odor acuity, greater reactivity to smells and also increased odor awareness compared to the healthy controls. Within the patients group, those suffering from JHS displayed higher functioning in all olfactory domains compared to the non-JHS ones. The JHS and anxiety measures emerged as predictor variables of the olfactory function. -

Neuropsychiatric Manifestations of COVID-19 Can Be Clustered in Three
www.nature.com/scientificreports OPEN Neuropsychiatric manifestations of COVID‑19 can be clustered in three distinct symptom categories Fatemeh Sadat Mirfazeli1,8, Atiye Sarabi‑Jamab2,8, Amin Jahanbakhshi3, Alireza Kordi4, Parisa Javadnia4, Seyed Vahid Shariat1, Oldooz Aloosh5, Mostafa Almasi‑Dooghaee6 & Seyed Hamid Reza Faiz7* Several studies have reported clinical manifestations of the new coronavirus disease. However, few studies have systematically evaluated the neuropsychiatric complications of COVID‑19. We reviewed the medical records of 201 patients with confrmed COVID‑19 (52 outpatients and 149 inpatients) that were treated in a large referral center in Tehran, Iran from March 2019 to May 2020. We used clustering approach to categorize clinical symptoms. One hundred and ffty‑one patients showed at least one neuropsychiatric symptom. Limb force reductions, headache followed by anosmia, hypogeusia were among the most common neuropsychiatric symptoms in COVID‑19 patients. Hierarchical clustering analysis showed that neuropsychiatric symptoms group together in three distinct groups: anosmia and hypogeusia; dizziness, headache, and limb force reduction; photophobia, mental state change, hallucination, vision and speech problem, seizure, stroke, and balance disturbance. Three non‑ neuropsychiatric cluster of symptoms included diarrhea and nausea; cough and dyspnea; and fever and weakness. Neuropsychiatric presentations are very prevalent and heterogeneous in patients with coronavirus 2 infection and these heterogeneous presentations may be originating from diferent underlying mechanisms. Anosmia and hypogeusia seem to be distinct from more general constitutional‑like and more specifc neuropsychiatric symptoms. Skeletal muscular manifestations might be a constitutional or a neuropsychiatric symptom. In December 2019 a number of severe acute respiratory syndrome (SARS) were reported in Wuhan, China that became eventually a pandemic infection with over 8 million reported cases until June 2020 1. -
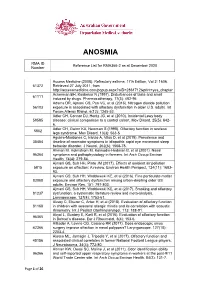
Reference List Concerning Anosmia
ANOSMIA RMA ID Reference List for RMA366-2 as at December 2020 Number Access Medicine (2008). Refractory asthma. 17th Edition, Vol 2: 1606. 61372 Retrieved 27 July 2011, from http://accessmedicine.com/popup.aspx?aID=2861712&print=yes_chapter Ackerman BH, Kasbekar N (1997). Disturbances of taste and smell 61111 induced by drugs. Pharmacotherapy, 17(3): 482-96. Adams DR, Ajmani GS, Pun VC, et al (2016). Nitrogen dioxide pollution 56103 exposure is associated with olfactory dysfunction in older U.S. adults. Int Forum Allergy Rhinol, 6(12): 1245-52. Adler CH, Connor DJ, Hentz JG, et al (2010). Incidental Lewy body 59585 disease: clinical comparison to a control cohort. Mov Disord, 25(5): 642- 6. Adler CH, Gwinn KA, Newman S (1998). Olfactory function in restless 5802 legs syndrome. Mov Disord, 13(3): 563-5. Aguirre-Mardones C, Iranzo A, Vilas D, et al (2015). Prevalence and 35454 timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol, 262(6): 1568-78. Ahman M, Holmstrom M, Kolmodin-Hedman B, et al (2001). Nasal 96364 symptoms and pathophysiology in farmers. Int Arch Occup Environ Health, 74(4): 279-84. Ajmani GS, Suh HH, Pinto JM (2017). Effects of ambient air pollution 5815 exposure on olfaction: A review. Environ Health Perspect, 124(11): 1683- 93. Ajmani GS, Suh HH, Wroblewski KE, et al (2016). Fine particulate matter 83088 exposure and olfactory dysfunction among urban-dwelling older US adults. Environ Res, 151: 797-803. Ajmani GS, Suh HH, Wroblewski KE, et al (2017). Smoking and olfactory 81237 dysfunction: a systematic literature review and meta-analysis. -

Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: 3 Prevalence, Severity, Timing and Associated Characteristics 4 5 Marlene M
Complete Manuscript Click here to access/download;Complete Manuscript;manuscript 042220 v3.docx This manuscript has been accepted for publication in Otolaryngology-Head and Neck Surgery. 2 Olfactory dysfunction and sinonasal symptomatology in COVID-19: 3 prevalence, severity, timing and associated characteristics 4 5 Marlene M. Speth, MD, MA1, Thirza Singer-Cornelius, MD1, Michael Obere, PhD2, Isabelle 6 Gengler, MD3, Steffi J. Brockmeier, MD1, Ahmad R. Sedaghat, MD, PhD3 7 8 9 1Klinik für Hals-, Nasen-, Ohren- Krankheiten, Hals-und Gesichtschirurgie, Kantonsspital 10 Aarau, Switzerland, 2Institute for Laboratory Medicine, Kantonsspital Aarau, Aarau, 11 Switzerland, 3Department of Otolaryngology—Head and Neck Surgery, University of 12 Cincinnati College of Medicine, Cincinnati, OH, USA. 13 14 15 Funding: MMS and TSC received funding from Kantonsspital Aarau, Department of 16 Otolaryngology, Funded by Research Council KSA 1410.000.128 17 18 Conflicts of Interest: None 19 20 21 Authors’ contributions: 22 MMS: designed and performed study, wrote and revised manuscript, approved final 23 manuscript. 24 TSC: designed and performed study, approved final manuscript. 25 MO: performed study, approved final manuscript. 26 IG: designed study, revised manuscript and approved final manuscript 27 SJB: designed and performed study, revised manuscript and approved final manuscript 28 ARS: conceived, designed and performed study, wrote and revised manuscript, approved 29 final manuscript. 30 31 32 Corresponding Author: 33 Ahmad R. Sedaghat, MD, PhD 34 -

Topamax® Tablets and Sprinkle Capsules Topiramate New Zealand Data Sheet
TOPAMAX® TABLETS AND SPRINKLE CAPSULES TOPIRAMATE NEW ZEALAND DATA SHEET 1. PRODUCT NAME TOPAMAX® 25 mg, 50 mg, 100 mg & 200 mg film-coated tablets TOPAMAX® Sprinkle 15 mg, 25 mg & 50 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION TABLETS Each tablet contains 25 mg, 50 mg, 100 mg or 200 mg of topiramate. Excipient(s) with known effect: Lactose monohydrate For a full list of excipients, see section 6.1. SPRINKLE CAPSULES Each capsule contains 15 mg, 25 mg or 50 mg of topiramate. Excipients with known effect: Sugar For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM TABLETS 25 mg: Round, white, film-coated tablets, marked “TOP” on one side and “25” on the other. 50 mg: Round, light-yellow, film-coated tablets, marked “TOP” on one side and “50” on the other 100 mg: Round, yellow, film-coated tablets, marked “TOP” on one side and “100” on the other 200 mg: Round, salmon, film-coated tablets, marked “TOP” on one side and “200” on the other. SPRINKLE CAPSULES Hard capsules enclosing small, white to off-white spheres. Each gelatin capsule consists of a clear (natural) capsule cap and a white capsule body. 15 mg: imprinted with “TOP” on cap and “15 mg” on body 25 mg: imprinted with “TOP” on cap and “25 mg” on body 50mg: imprinted with “TOP” on cap and “50mg” on body (not marketed). CCDS201005v23 1 TOPAMAX(201215)ADS 4. CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS EPILEPSY TOPAMAX is indicated in adults and children, 2 years and over: • as monotherapy in patients with newly diagnosed epilepsy • for conversion to monotherapy in patients with epilepsy • as add-on therapy in partial onset seizures, generalised tonic-clonic seizures or seizures associated with Lennox-Gastaut syndrome. -

Association of Olfactory and Pulmonary Function in Middle-Aged and Older Adults: the Korea National Health and Nutrition Examination Survey
Journal of Clinical Medicine Article Association of Olfactory and Pulmonary Function in Middle-Aged and Older Adults: The Korea National Health and Nutrition Examination Survey Ji-Sun Kim , Jun-Ook Park, Dong-Hyun Lee , Ki-Hong Chang and Byung Guk Kim * Department of Otolaryngology-Head and Neck Surgery, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 07345, Korea; [email protected] (J.-S.K.); [email protected] (J.-O.P.); [email protected] (D.-H.L.); [email protected] (K.-H.C.) * Correspondence: [email protected]; Tel.: +82-2-2030-4558 Abstract: Objectives: To identify the relationship between pulmonary function and subjective olfac- tory dysfunction in middle-aged and older adults. Materials and Methods: We used Korea National Health and Nutrition Examination Survey data from 2010 to 2012 to analyze 6191 participants in their 50s or older. Results: The frequency of olfactory dysfunction was 6.8% among the subjects with normal pulmonary function tests, but was significantly more frequent in those diagnosed with restrictive (9.6%) or obstructive (10.1%) pulmonary function. Forced volume vital capacity, forced expiratory volume (FEV)1, FEV6, and peak expiratory flow were significantly lower in the olfactory dysfunction group. The risk of olfactory dysfunction was significantly associated with obstructive pulmonary function (odds ratio (OR) [95% confidence interval (CI)]: 1.449 [1.010–2.081]) after ad- justing for confounders (sex, rhinitis, chronic rhinosinusitis, hypertension, dyslipidemia, education Citation: Kim, J.-S.; Park, J.-O.; Lee, D.-H.; Chang, K.-H.; Kim, B.G. level, stress, depressed mood, and suicidal ideation). -

Depression, Olfaction, and Quality of Life: a Mutual Relationship
brain sciences Review Depression, Olfaction, and Quality of Life: A Mutual Relationship Marion Rochet 1, Wissam El-Hage 1,2 ID , Sami Richa 3, François Kazour 1,4 ID and Boriana Atanasova 1,* ID 1 UMR 1253, iBrain, Université de Tours, Inserm, 37200 Tours, France; [email protected] (M.R.); [email protected] (W.E.-H.); [email protected] (F.K.) 2 CHRU de Tours, Clinique Psychiatrique Universitaire, 37044 Tours, France 3 Department of Psychiatry, Faculty of Medicine, Saint-Joseph University, P.O. Box 17-5208, 11-5076 Beirut, Lebanon; [email protected] 4 Psychiatric Hospital of the Cross, 60096 Jal Eddib, Lebanon * Correspondence: [email protected]; Tel.: +33-2-47-36-73-05 Received: 13 April 2018; Accepted: 3 May 2018; Published: 4 May 2018 Abstract: Olfactory dysfunction has been well studied in depression. Common brain areas are involved in depression and in the olfactory process, suggesting that olfactory impairments may constitute potential markers of this disorder. Olfactory markers of depression can be either state (present only in symptomatic phases) or trait (persistent after symptomatic remission) markers. This study presents the etiology of depression, the anatomical links between olfaction and depression, and a literature review of different olfactory markers of depression. Several studies have also shown that olfactory impairment affects the quality of life and that olfactory disorders can affect daily life and may be lead to depression. Thus, this study discusses the links between olfactory processing, depression, and quality of life. Finally, olfaction is an innovative research field that may constitute a new therapeutic tool for the treatment of depression. -

124 Epochs of Anosmia and Ageusia in Multiple Sclerosis
78 ABSTRACTS 2 Director, Head of Corporate Medical Affairs, H. 29.0 for patients with anxious distress in the adjunctive Lundbeck A/S, Valby, Denmark brexpiprazole (n = 462) group and 29.1 in the placebo 3 Director, Global Medical Affairs, Otsuka (n = 327) group; while those with anxious depression Pharmaceutical Development & Commercialization, were 28.9 (brexpiprazole; n = 384) and 28.6 (placebo; Inc., Princeton, NJ, USA n = 282). Compared to those receiving placebo, patients 4 Senior Director, Biostatistics, Otsuka Pharmaceutical with both anxious distress and anxious depression who Development & Commercialization, Inc., Princeton, received adjunctive brexpiprazole showed a greater NJ, USA 5 Senior Director, Global Medical Affairs, Otsuka improvement in MADRS total score (LS mean difference = = Pharmaceutical Development & Commercialization, -2.38, p 0.0001 and -1.68, p 0.012, respectively). Inc., Princeton, NJ, USA These improvements, compared to placebo, were similar to those in patients who had not met the criteria for ABSTRACT: Study objectives: Symptoms of anxiety are anxious distress (-1.40, p = 0.023) or anxious depression prevalent in Major Depressive Disorder (MDD) and are (-2.17, p < 0.001). associated with greater illness severity, suicidality, impaired functioning and poor response to antidepres- CONCLUSION: Adjunctive brexpiprazole may be effica- sant treatment (ADT). In MDD, anxiety symptoms can be cious in reducing depressive symptoms both in patients assessed as ‘anxious distress’ (new DSM-5 specifier) or with or without symptoms of anxiety. ‘anxious depression’ (score ≥7 on the HAM-D anxiety/ FUNDING ACKNOWLEDGEMENTS: The studies were funded somatization factor). Brexpiprazole is a serotonin– by H. Lundbeck A/S and Otsuka Pharmaceutical Devel- dopamine activity modulator that is a partial agonist at opment & Commercialization, Inc. -

Anosmia but Not Ageusia As a COVID-19-Related Symptom Among Cancer Patients—First Results from the PAPESCO-19 Cohort Study
cancers Article Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study Ke Zhou 1,*, Audrey Blanc-Lapierre 2, Valérie Seegers 2, Michèle Boisdron-Celle 3, Frédéric Bigot 4, Marianne Bourdon 1,5, Hakim Mahammedi 6, Aurélien Lambert 7 , Mario Campone 8, Thierry Conroy 7 , Frédérique Penault-Llorca 9, Martine M. Bellanger 1,10 and Jean-Luc Raoul 8 1 Department of Human and Social Sciences, Institut de Cancérologie de l’Ouest (ICO), 44805 Saint-Herblain, France; [email protected] (M.B.); [email protected] (M.M.B.) 2 Department of Biostatistic, Institut de Cancérologie de l’Ouest, 44805 Saint-Herblain, France; [email protected] (A.B.-L.); [email protected] (V.S.) 3 Department of Biopathology, Institut de Cancérologie de l’Ouest, 49055 Angers, France; [email protected] 4 Department of Medical Oncology, Institut de Cancérologie de l’Ouest, 49055 Angers, France; [email protected] 5 Research Unit UMR INSERM 1246 SPHERE, Universités de Nantes et Tours, 44035 Nantes, France 6 Department of Medical Oncology, Centre Jean Perrin, 63011 Clermont-Ferrand, France; [email protected] 7 Department of Medical Oncology, Institut de Cancérologie de Lorraine, 54511 Vandoeuvre-lès-Nancy, France; Citation: Zhou, K.; Blanc-Lapierre, [email protected] (A.L.); [email protected] (T.C.) A.; Seegers, V.; Boisdron-Celle, M.; 8 Department of Medical Oncology, Institut de Cancérologie de l’Ouest, 44805 Saint-Herblain, France; Bigot, F.; Bourdon, M.; Mahammedi, [email protected] (M.C.); [email protected] (J.-L.R.) H.; Lambert, A.; Campone, M.; 9 Department of Biopathology and INSERM U1240, Centre Jean Perrin, 63011 Clermont-Ferrand, France; Conroy, T.; et al.