Management of Septic Bursitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iliopsoas Tendonitis/Bursitis Exercises
ILIOPSOAS TENDONITIS / BURSITIS What is the Iliopsoas and Bursa? The iliopsoas is a muscle that runs from your lower back through the pelvis to attach to a small bump (the lesser trochanter) on the top portion of the thighbone near your groin. This muscle has the important job of helping to bend the hip—it helps you to lift your leg when going up and down stairs or to start getting out of a car. A fluid-filled sac (bursa) helps to protect and allow the tendon to glide during these movements. The iliopsoas tendon can become inflamed or overworked during repetitive activities. The tendon can also become irritated after hip replacement surgery. Signs and Symptoms Iliopsoas issues may feel like “a pulled groin muscle”. The main symptom is usually a catch during certain movements such as when trying to put on socks or rising from a seated position. You may find yourself leading with your other leg when going up the stairs to avoid lifting the painful leg. The pain may extend from the groin to the inside of the thigh area. Snapping or clicking within the front of the hip can also be experienced. Do not worry this is not your hip trying to pop out of socket but it is usually the iliopsoas tendon rubbing over the hip joint or pelvis. Treatment Conservative treatment in the form of stretching and strengthening usually helps with the majority of patients with iliopsoas bursitis. This issue is the result of soft tissue inflammation, therefore rest, ice, anti- inflammatory medications, physical therapy exercises, and/or injections are effective treatment options. -
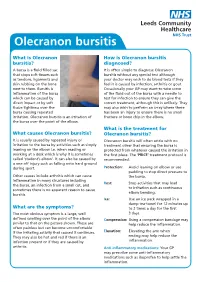
Olecranon Bursitis
Olecranon bursitis What is Olecranon How is Olecranon bursitis bursitis? diagnosed? A bursa is a fluid filled sac It is often simple to diagnose Olecranon that stops soft tissues such bursitis without any special test although as tendons, ligaments and your doctor may wish to do blood tests if they skin rubbing on the bone feel it is caused by infection, arthritis or gout. next to them. Bursitis is Occasionally your GP may want to take some inflammation of the bursa of the fluid out of the bursa with a needle to which can be caused by test for infection to ensure they can give the direct impact or by soft correct treatment, although this is unlikely. They tissue tightness over the may also wish to perform an x-ray where there bursa causing repeated has been an injury to ensure there is no small irritation. Olecranon bursitis is an irritation of fracture or bone chip in the elbow. the bursa over the point of the elbow. What is the treatment for What causes Olecranon bursitis? Olecranon bursitis? It is usually caused by repeated injury or Olecranon bursitis will often settle with no irritation to the bursa by activities such as simply treatment other that ensuring the bursa is leaning on the elbow i.e. when reading or protected from whatever caused the irritation in working at a desk which is why it is sometimes the first place. The ‘PRICE’ treatment protocol is called ‘student’s elbow’. It can also be caused by recommended: a one off injury such as falling onto hard ground during sport. -

OES Site Color Scheme 1
Nuisance Problems You will Grow to Love Thomas V Gocke, MS, ATC, PA-C, DFAAPA President & Founder Orthopaedic Educational Services, Inc. Boone, NC [email protected] www.orthoedu.com Orthopaedic Educational Services, Inc. © 2016 Orthopaedic Educational Services, Inc. all rights reserved. Faculty Disclosures • Orthopaedic Educational Services, Inc. Financial Intellectual Property No off label product discussions American Academy of Physician Assistants Financial PA Course Director, PA’s Guide to the MSK Galaxy Urgent Care Association of America Financial Intellectual Property Faculty, MSK Workshops Ferring Pharmaceuticals Consultant Orthopaedic Educational Services, Inc. © 2016 Orthopaedic Educational Services, Inc. all rights reserved. 2 LEARNING GOALS At the end of this sessions you will be able to: • Recognize nuisance conditions in the Upper Extremity • Recognize nuisance conditions in the Lower Extremity • Recognize common Pediatric Musculoskeletal nuisance problems • Recognize Radiographic changes associates with common MSK nuisance problems • Initiate treatment plans for a variety of MSK nuisance conditions Orthopaedic Educational Services, Inc. © 2016 Orthopaedic Educational Services, Inc. all rights reserved. Inflammatory Response Orthopaedic Educational Services, Inc. © 2016 Orthopaedic Educational Services, Inc. all rights reserved. Inflammatory Response* When does the Inflammatory response occur: • occurs when injury/infection triggers a non-specific immune response • causes proliferation of leukocytes and increase in blood flow secondary to trauma • increased blood flow brings polymorph-nuclear leukocytes (which facilitate removal of the injured cells/tissues), macrophages, and plasma proteins to injured tissues *Knight KL, Pain and Pain relief during Cryotherapy: Cryotherapy: Theory, Technique and Physiology, 1st edition, Chattanooga Corporation, Chattanooga, TN 1985, p 127-137 Orthopaedic Educational Services, Inc. © 2016 Orthopaedic Educational Services, Inc. -

Sports Medicine Examination Outline
Sports Medicine Examination Content I. ROLE OF THE TEAM PHYSICIAN 1% A. Ethics B. Medical-Legal 1. Physician responsibility 2. Physician liability 3. Preparticipation clearance 4. Return to play 5. Waiver of liability C. Administrative Responsibilities II. BASIC SCIENCE OF SPORTS 16% A. Exercise Physiology 1. Training Response/Physical Conditioning a.Aerobic b. Anaerobic c. Resistance d. Flexibility 2. Environmental a. Heat b.Cold c. Altitude d.Recreational diving (scuba) 3. Muscle a. Contraction b. Lactate kinetics c. Delayed onset muscle soreness d. Fiber types 4. Neuroendocrine 5. Respiratory 6. Circulatory 7. Special populations a. Children b. Elderly c. Athletes with chronic disease d. Disabled athletes B. Anatomy 1. Head/Neck a.Bone b. Soft tissue c. Innervation d. Vascular 2. Chest/Abdomen a.Bone b. Soft tissue c. Innervation d. Vascular 3. Back a.Bone b. Soft tissue c. Innervation 1 d. Vascular 4. Shoulder/Upper arm a. Bone b. Soft tissue c. Innervation d. Vascular 5. Elbow/Forearm a. Bone b. Soft tissue c. Innervation d. Vascular 6. Hand/Wrist a. Bone b. Soft tissue c. Innervation d. Vascular 7. Hip/Pelvis/Thigh a. Bone b. Soft tissue c. Innervation d. Vascular 8. Knee a. Bone b. Soft tissue c. Innervation d. Vascular 9. Lower Leg/Foot/Ankle a. Bone b. Soft tissue c. Innervation d. Vascular 10. Immature Skeleton a. Physes b. Apophyses C. Biomechanics 1. Throwing/Overhead activities 2. Swimming 3. Gait/Running 4. Cycling 5. Jumping activities 6. Joint kinematics D. Pharmacology 1. Therapeutic Drugs a. Analgesics b. Antibiotics c. Antidiabetic agents d. Antihypertensives e. -

Pes Anserine Bursitis
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Pes Anserine Bursitis ICD 9 Codes: 726.61 Case Type / Diagnosis: The pes anserine bursa lies behind the medial hamstring, which is composed of the tendons of the sartorius, gracilis and semitendinosus (SGT) muscles. Because these 3 tendons splay out on the anterior aspect of the tibia and give the appearance of the foot of a goose, pes anserine bursitis is also known as goosefoot bursitis.1 These muscles provide for medial stabilization of the knee by acting as a restraint to excessive valgus opening. They also provide a counter-rotary torque function to the knee joint. The pes anserine has an eccentric role during the screw-home mechanism that dampens the effect of excessively forceful lateral rotation that may accompany terminal knee extension.2 Pes anserine bursitis presents as pain, tenderness and swelling over the anteromedial aspect of the knee, 4 to 5 cm below the joint line.3 Pain increases with knee flexion, exercise and/or stair climbing. Inflammation of this bursa is common in overweight, middle-aged women, and may be associated with osteoarthritis of the knee. It also occurs in athletes engaged in activities such as running, basketball, and racquet sports.3 Other risk factors include: 1 • Incorrect training techniques, or changes in terrain and/or distanced run • Lack of flexibility in hamstring muscles • Lack of knee extension • Patellar malalignment Indications for Treatment: • Knee Pain • Knee edema • Decreased active and /or passive ROM of lower extremities • Biomechanical dysfunction lower extremities • Muscle imbalances • Impaired muscle performance (focal weakness or general conditioning) • Impaired function Contraindications: • Patients with active signs/symptoms of infection (fever, chills, prolonged and obvious redness or swelling at hip joint). -

Gluteal Tendinopathy
Gluteal Tendinopathy What is a Gluteal Tendinopathy? In lying Up until recently hip bursitis was diagnosed as the main Either on your bad hip or with bad cause of lateral hip pain but recent studies suggest that an hip hanging across body like so irritation of the gluteus muscle tendon is the likeliest cause. The tendon attaches onto a bony prominence (greater trochanter) and it is here that the tendon is subject to All these positions lead to increase friction of the tendon, compressive forces leading to irritation. can cause pain and slow the healing process. This can result in pain over the lateral hip which can refer down the outside For sleeping you might like to try these positions: of the thigh and into the knee. How common is it? Gluteal tendinopathy is relatively common affecting 10-25% of the population. It is 3 times more prevalent in women than men and is most common in women between the ages of 40 and 60. One of the reasons for this is women It is also important to modify your activity. Avoid or reduce tend to have a greater angle at their hip joint increasing things that flare up your pain, this could be climbing stairs compressive forces on the tendon. or hills or those longer walks/runs. Signs and Symptoms Exercise Therapy • Pain on the outside of your hip, can refer down outside of the thigh to the knee This is best administered by a Physiotherapist to suit the • Worse when going up and/or down stairs individual but below is a rough guide to exercises which • Worse lying on affected side (and sometimes on the can help a gluteal tendinopathy. -

Bilateral Calcified Ischiogluteal Bursitis and Shoulder Tendinopathy
Bilateral Calcified Ischiogluteal Bursitis and Conflict of Interest: None Shoulder Tendinopathy: A Case Report declared Seyyed-Mohsen Hosseininejad1,2, Saman Shakeri1, Hossein Mohebbi1, Mehdi Aarabi2, Shiva Momen3 This article has been peer reviewed. 1Shahid Beheshti University of Medical Sciences, Tehran, Iran 2 Golestan University of Medical Sciences, Gorgan, Iran Article Submitted on: 21st 3Mazandaran University of Medical Sciences, Sari, Iran January 2019 Article Accepted on: 1st ABSTRACT June 2020 The ischiogluteal bursitis which is a rare the buttock. Ischiogluteal bursitis Aspiration Funding Sources: None disorder is irregularly found between the showed calcareous deposits; local injection declared gluteus maximus and ischial tuberosity. A of corticosteroid helped the patient to get 41-year-old female with bilateral calcifying free of symptoms. Calcified ischiogluteal Correspondence to: Dr ischiogluteal bursitis and her right shoulder bursitis is a rare condition but simply Seyyed-Mohsen Hosseininejad tendinopathy were presented. She had no diagnosed on x-ray. Local steroid injection related past medical history nor trauma to could provide symptom relief. Address: Shahid Beheshti University of Medical Sciences, Tehran, Iran Keywords: Calcifying Ischiogluteal Bursitis; Aspiration; Treatment; Shoulder pain; Tendinopathy E-mail: Hosseininejad.s.mohsen INTRODUCTION painful swelling in her both buttocks. The patient @gmail.com Ischiogluteal bursitis is a rare condition in which had no related past medical history nor recent Cite this Article: bursa between the gluteus maximus muscle and major trauma. Ischial tuberosities had swelling Hosseininejad SM, ischial tuberosity, which physiologically and tenderness in. Right shoulder had positive Shakeri S, Mohebbi H, decreases the frictional force, develops impingement tests but full range of motion. Aarabi M, Momen S. -

Endoscopic Hamstring Repair
Lorem Ipsum Endoscopic Hamstring Repair Carlos A. Guanche, MD Southern California Orthopedic Institute 12 Lorem Ipsum 2 Endoscopic Hamstring Repair With the expansion of knowledge regarding hip pathologies as a result of the increased treatment of hip problems arthroscopically has come an expanded treatment of many injuries that were previously treated through open methods. The treatment of symptomatic ischial bursitis and hamstring injuries is one such area. In this paper, the author describes the surgical procedure and discusses the findings and preliminary outcomes in a group of the first 15 patients undergoing the procedure. The clinical rationale associated with the treatment algorithm is also discussed. Hamstring injuries have been effectively addressed in the past with a variety of open methods.(1,2) However, the endoscopic management of much pathology previously treated with more invasive, open approaches has evolved. The technique described in this chapter is another such evolution. Hamstring injuries are common and can affect all levels of The hamstrings originate from the ischial tuberosity and athletes. (3-7) There is a continuum of hamstring injuries insert distally below the knee on the proximal tibia, with the that can range from musculotendinous strains to avulsion exception of the short head of the biceps femoris. The tibial injuries. (3,4) Most hamstring strains do not require surgical branch of the sciatic nerve innervates the semitendinosus, intervention and resolve with a variety of modalities and semimembranosus, and the peroneal branch of the sciatic rest. (3-7) In some patients, chronic pain can occur at the nerve innervates the long head of the biceps femoris.(5) hamstring origin from either partial or complete tears as well as from chronic ischial bursitis. -

An Unusual Klebsiella Septic Bursitis Mimicking a Soft Tissue Tumor
Case Report Eur J Gen Med 2013;10(1):47-50 An Unusual Klebsiella Septic Bursitis Mimicking a Soft Tissue Tumor Mehmet Ali Acar1, Nazım Karalezli2, Ali Güleç3 ABSTRACT Because of its subcutaneous location prepatellar bursitis is frequently complicated by an infection. Gram-positive organisms, primarily Staphylococcus aureus account for the majority of cases of septic bursitis. Local cutaneous trauma can lead to direct inoculation of the bursa with normal skin flora in patients with occupations, such as mechanics, carpenters and farmers. A 71-year- old male was admitted to our department with a history of pain and swelling of his right knee over a 20 year period. Physical examination revealed a swollen, suppurative mass with ulceration of the skin and local erythema which mimicked a soft tissue tumor at first sight. Magnetic resonance imaging of the knee revealed a 13*12*10cm well-circumscribed, septated, capsulated, fluid-filled prepatellar bursa without evidence of tendinous or muscular invasion. The mass was excised en bloc, including the bursa and the overlying skin. The defect was closed with a split thickness skin graft. The patient had 100 degrees flexion and full extension after 45 days postoperatively, and he continued to work as a farmer. Key words: Klebsiella, septic bursitis, haemorrhage, mass Yumuşak Doku Tümöre Benzeyen Nadir Klebsiella Septik Bursiti ÖZET ilt altı yerleşiminden dolayı prepatellar bursitlerde enfeksiyon görülmesi sık olur. Gram pozitif mikroorganizmalar, özellikle stafilokokkus aureus en sık etkendir. Tamirciler, halıcılar ve çiftçiler gibi travmaya çok maruz kalan meslek gruplarında direk olarak etken cilt florasından bursaya ulaşabilir. 71 yaşında erkek hasta 20 yılı aşan ağrı ve şişlik nedeni ile kliniğimize başvurdu. -

The Anatomy of the Deep Infrapatellar Bursa of the Knee Robert F
0363-5465/98/2626-0129$02.00/0 THE AMERICAN JOURNAL OF SPORTS MEDICINE, Vol. 26, No. 1 © 1998 American Orthopaedic Society for Sports Medicine The Anatomy of the Deep Infrapatellar Bursa of the Knee Robert F. LaPrade,* MD Department of Orthopaedic Surgery, University of Minnesota, Minneapolis, Minnesota ABSTRACT knee joint, and to define a consistent surgical approach to the deep infrapatellar bursa. Disorders of the deep infrapatellar bursa are important to include in the differential diagnosis of anterior knee pain. Knowledge regarding its anatomic location can MATERIALS AND METHODS aid the clinician in establishing a proper diagnosis. Fifty cadaveric knees were dissected, and the deep infrapa- Thorough dissections of the anterior aspect of the knee of tellar bursa had a consistent anatomic location in all 50 nonpaired cadaveric knees were performed. There were specimens. The deep infrapatellar bursa was located 27 male and 23 female cadaveric knees with 25 right and directly posterior to the distal 38% of the patellar ten- 25 left knees. The average age of the specimens was 71.8 don, just proximal to its insertion on the tibial tubercle. years (range, 42 to 93). After the skin and subcutaneous There was no communication to the knee joint. Its tissues of the anterior aspect of the knee were carefully average width at the most proximal margin of the tibial dissected away, an approach to the deep infrapatellar tubercle was slightly wider than the average distal bursa of the knee was made through medial and lateral width of the patellar tendon. It was found to be partially arthrotomy incisions along the patella, followed by compartmentalized, with a fat pad apron extending transection of the quadriceps tendon from the patella. -
Orthopaedics Instructions: to Best Navigate the List, First Download This PDF File to Your Computer
Orthopaedics Instructions: To best navigate the list, first download this PDF file to your computer. Then navigate the document using the bookmarks feature in the left column. The bookmarks expand and collapse. Finally, ensure that you look at the top of each category and work down to review notes or specific instructions. Bookmarks: Bookmarks: notes or specific with expandable instructions and collapsible topics As you start using the codes, it is recommended that you also check in Index and Tabular lists to ensure there is not a code with more specificity or a different code that may be more appropriate for your patient. Copyright APTA 2016, ALL RIGHTS RESERVED. Last Updated: 09/14/16 Contact: [email protected] Orthopaedics Disorder by site: Ankle Achilles tendinopathy ** Achilles tendinopathy is not listed in ICD10 M76.6 Achilles tendinitis Achilles bursitis M76.61 Achilles tendinitis, right leg M76.62 Achilles tendinitis, left leg ** Tendinosis is not listed in ICD10 M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, excluding foot M76.892 Other specified enthesopathies of left lower limb, excluding foot Posterior tibialis dysfunction **Posterior Tibial Tendon Dysfunction (PTTD) is not listed in ICD10 M76.82 Posterior tibial tendinitis M76.821 Posterior tibial tendinitis, right leg M76.822 Posterior tibial tendinitis, left leg M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, -

Download Versus Arthritis
Elbow pain Elbow pain information booklet Contents How does the elbow work? 4 What causes elbow pain and stiffness? 6 Should I see a healthcare professional? 8 What can I do to help myself? 9 How are elbow problems diagnosed? 12 What treatments are there for elbow pain? 14 Specific elbow conditions 18 Glossary 26 Research and new developments 27 Keeping active with elbow pain 28 Where can I find out more? 32 We’re the 10 million people living with arthritis. We’re the carers, researchers, health professionals, friends and parents all united in Talk to us 33 our ambition to ensure that one day, no one will have to live with the pain, fatigue and isolation that arthritis causes. We understand that every day is different. We know that what works for one person may not help someone else. Our information is a collaboration of experiences, research and facts. We aim to give you everything you need to know about your condition, the treatments available and the many options you can try, so you can make the best and most informed choices for your lifestyle. We’re always happy to hear from you whether it’s with feedback on our information, to share your story, or just to find out more about the work of Versus Arthritis. Contact us at [email protected] Words shown are explained in the glossary on p.26. Registered office: Versus Arthritis, Copeman House, St Mary’s Gate, Chesterfield S41 7TD in bold Registered Charity England and Wales No. 207711, Scotland No. SC041156.