Seminario Transdisciplinario Cinvestav
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

When Hiking Through Latin America, Be Alert to Chagas' Disease
When Hiking Through Latin America, Be Alert to Chagas’ Disease Geographical distribution of main vectors, including risk areas in the southern United States of America INTERNATIONAL ASSOCIATION 2012 EDITION FOR MEDICAL ASSISTANCE For updates go to www.iamat.org TO TRAVELLERS IAMAT [email protected] www.iamat.org @IAMAT_Travel IAMATHealth When Hiking Through Latin America, Be Alert To Chagas’ Disease COURTESY ENDS IN DEATH segment upwards, releases a stylet with fine teeth from the proboscis and Valle de los Naranjos, Venezuela. It is late afternoon, the sun is sinking perforates the skin. A second stylet, smooth and hollow, taps a blood behind the mountains, bringing the first shadows of evening. Down in the vessel. This feeding process lasts at least twenty minutes during which the valley a campesino is still tilling the soil, and the stillness of the vinchuca ingests many times its own weight in blood. approaching night is broken only by a light plane, a crop duster, which During the feeding, defecation occurs contaminating the bite wound periodically flies overhead and disappears further down the valley. with feces which contain parasites that the vinchuca ingested during a Bertoldo, the pilot, is on his final dusting run of the day when suddenly previous bite on an infected human or animal. The irritation of the bite the engine dies. The world flashes before his eyes as he fights to clear the causes the sleeping victim to rub the site with his or her fingers, thus last row of palms. The old duster rears up, just clipping the last trees as it facilitating the introduction of the organisms into the bloodstream. -

Vectors of Chagas Disease, and Implications for Human Health1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Jurberg Jose, Galvao Cleber Artikel/Article: Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health 1095-1116 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health1 J. JURBERG & C. GALVÃO Abstract: The members of the subfamily Triatominae (Heteroptera, Reduviidae) are vectors of Try- panosoma cruzi (CHAGAS 1909), the causative agent of Chagas disease or American trypanosomiasis. As important vectors, triatomine bugs have attracted ongoing attention, and, thus, various aspects of their systematics, biology, ecology, biogeography, and evolution have been studied for decades. In the present paper the authors summarize the current knowledge on the biology, ecology, and systematics of these vectors and discuss the implications for human health. Key words: Chagas disease, Hemiptera, Triatominae, Trypanosoma cruzi, vectors. Historical background (DARWIN 1871; LENT & WYGODZINSKY 1979). The first triatomine bug species was de- scribed scientifically by Carl DE GEER American trypanosomiasis or Chagas (1773), (Fig. 1), but according to LENT & disease was discovered in 1909 under curi- WYGODZINSKY (1979), the first report on as- ous circumstances. In 1907, the Brazilian pects and habits dated back to 1590, by physician Carlos Ribeiro Justiniano das Reginaldo de Lizárraga. While travelling to Chagas (1879-1934) was sent by Oswaldo inspect convents in Peru and Chile, this Cruz to Lassance, a small village in the state priest noticed the presence of large of Minas Gerais, Brazil, to conduct an anti- hematophagous insects that attacked at malaria campaign in the region where a rail- night. -

Trypanosoma Cruzi in Latin America.', Plos Neglected Tropical Diseases., 14 (8)
Durham Research Online Deposited in DRO: 23 August 2020 Version of attached le: Published Version Peer-review status of attached le: Peer-reviewed Citation for published item: Bender, Andreas and Python, Andre and Lindsay, Steve W. and Golding, Nick and Moyes, Catherine L. (2020) 'Modelling geospatial distributions of the triatomine vectors of Trypanosoma cruzi in Latin America.', PLoS neglected tropical diseases., 14 (8). e0008411. Further information on publisher's website: https://doi.org/10.1371/journal.pntd.0008411 Publisher's copyright statement: Copyright: c 2020 Bender et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk PLOS NEGLECTED TROPICAL DISEASES RESEARCH ARTICLE Modelling geospatial distributions of the triatomine vectors of Trypanosoma cruzi in Latin America 1¤ 1 2 3 Andreas BenderID *, Andre Python , Steve W. -

Triatoma Infestans in the Bolivian Chaco Using a Microencapsulated Insecticide Formulation David Eladio Gorla1*, Roberto Vargas Ortiz2 and Silvia Susana Catalá1
Gorla et al. Parasites & Vectors (2015) 8:255 DOI 10.1186/s13071-015-0762-0 RESEARCH Open Access Control of rural house infestation by Triatoma infestans in the Bolivian Chaco using a microencapsulated insecticide formulation David Eladio Gorla1*, Roberto Vargas Ortiz2 and Silvia Susana Catalá1 Abstract Background: Triatoma infestans, the main vector of Trypanosoma cruzi (causative agent of Chagas disease) has been successfully eliminated over much of its original geographic distribution over the southern cone countries of South America. However, populations of the species are still infesting houses of rural communities of the Gran Chaco region of Argentina and Bolivia. This study reports for the first time a large-scale effect of a vector control intervention using a microencapsulated formulation of organophosphates and insect growth regulator on house infestation by T. infestans, in the southwestern region of Santa Cruz de la Sierra Department, within the Bolivian chaco. Methods: The vector control intervention included the treatment and entomological evaluation of 1626 individually coded and georeferenced houses with the microencapsulated formulation. House infestation by T. infestans was evaluated by active searches with fixed capture effort carried out before and after two, 16 and 32 months of the treatment application. Results: House infestation prevalence was 30.5% before the intervention, spatially aggregated in two clusters of 38 and 25 localities that showed 41% and 38% house infestation by T. infestans. Infestation prevalence was reduced to 2.4% two months after the intervention and remained very low (1.7%) until the end of the study after 32 months of the control intervention, without any other additional vector control intervention. -

Indoor Residual Spraying Practices Against Triatoma Infestans in The
Gonçalves et al. Parasites Vectors (2021) 14:327 https://doi.org/10.1186/s13071-021-04831-1 Parasites & Vectors RESEARCH Open Access Indoor residual spraying practices against Triatoma infestans in the Bolivian Chaco: contributing factors to suboptimal insecticide delivery to treated households Raquel Gonçalves1, Rhiannon A. E. Logan2, Hanafy M. Ismail2, Mark J. I. Paine2, Caryn Bern3 and Orin Courtenay1* Abstract Background: Indoor residual spraying (IRS) of insecticides is a key method to reduce vector transmission of Trypano- soma cruzi, causing Chagas disease in a large part of South America. However, the successes of IRS in the Gran Chaco region straddling Bolivia, Argentina, and Paraguay, have not equalled those in other Southern Cone countries. Aims: This study evaluated routine IRS practices and insecticide quality control in a typical endemic community in the Bolivian Chaco. Methods: Alpha-cypermethrin active ingredient (a.i.) captured onto flter papers ftted to sprayed wall surfaces, and in prepared spray tank solutions, were measured using an adapted Insecticide Quantifcation Kit (IQK™) validated against HPLC quantifcation methods. The data were analysed by mixed-efects negative binomial regression models to examine the delivered insecticide a.i. concentrations on flter papers in relation to the sprayed wall heights, spray coverage rates (surface area / spray time [m2/min]), and observed/expected spray rate ratios. Variations between health workers and householders’ compliance to empty houses for IRS delivery were also evaluated. Sedimentation rates of alpha-cypermethrin a.i. post-mixing of prepared spray tanks were quantifed in the laboratory. Results: Substantial variations were observed in the alpha-cypermethrin a.i. concentrations delivered; only 10.4% (50/480) of flter papers and 8.8% (5/57) of houses received the target concentration of 50 mg 20% a.i./m2. -

Elucidating the Mechanism of Trypanosoma Cruzi Acquisition by Triatomine Insects: Evidence from a Large Field Survey of Triatoma Infestans
Tropical Medicine and Infectious Disease Article Elucidating the Mechanism of Trypanosoma cruzi Acquisition by Triatomine Insects: Evidence from a Large Field Survey of Triatoma infestans Aaron W. Tustin 1, Ricardo Castillo-Neyra 2,3 , Laura D. Tamayo 2, Renzo Salazar 2 , Katty Borini-Mayorí 2 and Michael Z. Levy 2,3,* 1 Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA; [email protected] 2 Zoonotic Disease Research Lab, One Health Unit, School of Public Health and Administration, Universidad Peruana Cayetano Heredia, Lima, Lima Province 15102, Peru; [email protected] (R.C.-N.); [email protected] (L.D.T.); [email protected] (R.S.); [email protected] (K.B.-M.) 3 Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, PA 19104, USA * Correspondence: [email protected]; Tel.: +1-215-746-8131 Received: 25 March 2020; Accepted: 26 May 2020; Published: 1 June 2020 Abstract: Blood-sucking triatomine bugs transmit the protozoan parasite Trypanosoma cruzi, the etiologic agent of Chagas disease. We measured the prevalence of T. cruzi infection in 58,519 Triatoma infestans captured in residences in and near Arequipa, Peru. Among bugs from infected colonies, T. cruzi prevalence increased with stage from 12% in second instars to 36% in adults. Regression models demonstrated that the probability of parasite acquisition was roughly the same for each developmental stage. Prevalence increased by 5.9% with each additional stage. We postulate that the probability of acquiring the parasite may be related to the number of feeding events. -

Triatoma Rubrovaria
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 98(3): 367-371, April 2003 367 Triatoma rubrovaria (Blanchard, 1843) (Hemiptera-Reduviidae- Triatominae) III: Patterns of Feeding, Defecation and Resistance to Starvation Carlos Eduardo Almeida/**/+, Cesar Nascimento Francischetti, Raquel S Pacheco*, Jane Costa Núcleo de Informatização, Coleção Entomológica, Departamento de Entomologia *Laboratório de Sistemática Bioquímica, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brasil **Núcleo Avançado de Estudos de Vetores e Artrópodes Peçonhentos, Museu de Ciências Naturais, Centro Universitário de Barrra Mansa, UBM, Barra Mansa, RJ, Brasil Data from the Chagas Disease Control Program indicate a growing domiciliary and peridomiciliary invasion of Triatoma rubrovaria in the State of Rio Grande do Sul, where it has become the most frequent triatomine species captured there since the control of T. infestans. Bionomic characteristics that could influence the vectorial capacity of T. rubrovaria as vector of Trypanosoma cruzi were evaluated: patterns of (i) feeding, (ii) defecation, and (iii) resistance to starvation, using insects fed on mice. Fifty three percent of the females showed a defecation pattern conducive to chagasic transmission, defecating either on or near the bite site. The averages of the resistance to starvation varied from 48.1 to 179 days, for the first and fifth nymphal stages, respectively. Our study shows that with respect to the patterns of feeding, defecation and resistance to fasting, T. rubrovaria presented similar rates to the ones observed for other effective vector species, such as T. infestans. Thus, based on our studies we conclude that T. rubrovaria has biological characteristics that can positively influence its capacity to become infected and trans- mit T. -

2005 Ecology of Infectious Disease Program Review and Evaluation
National Institutes of Health National Institute of General Medical Sciences John E. Fogarty International Center National Science Foundation Directorate for Biological Sciences Directorate for Geosciences REVIEW OF THE JOINT NATIONAL INSTITUTES OF HEALTH / NATIONAL SCIENCE FOUNDATION ECOLOGY OF INFECTIOUS DISEASE PROGRAM Final Report July 18th – 20th, 2005 Fogarty International Center National Institutes of Health Bethesda, Maryland USA Expert Review Panelists: Joseph Bunnell, Ph.D. Donald Burke, M.D. (Chair) James Collins, Ph.D. Stephen Morse, Ph.D. Lee Riley, M.D. Estelle Russek-Cohen, Ph.D. Juli Trtanj, M.E.M. 1 Letter of Transmittal 2 Executive Summary Initiated in 1999, the Ecology of Infectious Diseases (EID) initiative is a competitive research grant program administered jointly by the National Science Foundation (NSF) and the Fogarty International Center (FIC). Its purpose is to encourage development of predictive models and discovery of principles for relationships between anthropogenic environmental change and transmission of infectious agents. In 2005, as part of its ongoing program review procedures, the Fogarty International Center (FIC) convened a panel of seven experts to review the achievements of the EID program to date and to make recommendations about its future. Fields of expertise represented on the panel included infectious diseases, epidemiology, public health, ecology, environmental science, and biostatistics. The panel met June 18th–20th, 2005, on the NIH campus in Bethesda, Maryland. Panel members conducted interviews in- person and via telephone with principal investigators on EID grants, key personnel on EID-funded projects, key staff members of NSF and NIH program partner agencies, EID program officers, and outside experts with relevant knowledge. -

Insecticide Resistance of Triatoma Infestans
Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia Frédéric Lardeux, Stéphanie Depickère, Stéphane Duchon, Tamara Chavez To cite this version: Frédéric Lardeux, Stéphanie Depickère, Stéphane Duchon, Tamara Chavez. Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia. Tropical Medicine and International Health, Wiley-Blackwell, 2010, 15 (9), pp.1037-1048. 10.1111/j.1365- 3156.2010.02573.x. hal-01254858 HAL Id: hal-01254858 https://hal.archives-ouvertes.fr/hal-01254858 Submitted on 12 Jan 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Post-print document. Tropical Medicine and International Health - 2010 - Volume 15, Issue 9, pages 1037–1048 Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia. Frédéric Lardeux 1, Stéphanie Depickère2, Stéphane Duchon1, Tamara Chavez2 1 Institut de Recherche pour le Développement (IRD), Montpellier, France 2 Laboratorio de Entomología Médica, Instituto Nacional de Laboratorios de Salud (INLASA), La Paz, Bolivia Running head: Insecticide resistance of T. infestans in Bolivia Corresponding author: Frédéric Lardeux, IRD-LIN, 911 avenue Agropolis, 34394 Montpellier Cedex 5, France. Tel: (+33) 4 67 41 32 22. -

Triatoma Sanguisuga, Eastern Blood-Sucking Conenose Bug (Hemiptera: Reduviidae) Chris Carlton, Forest Huval and T.E
Triatoma sanguisuga, Eastern Blood-Sucking Conenose Bug (Hemiptera: Reduviidae) Chris Carlton, Forest Huval and T.E. Reagan Description a professional insect diagnostician. At least nine members Adults of the eastern blood-sucking conenose bug of the subfamily Triatominae occur in the U.S., most in the are relatively large insects three-quarters of an inch to genus Triatoma, with additional species in Central and South seven-eighths of an inch (18 to 22 mm) in body length. America. The eastern blood-sucking conenose bug, Triatoma The body shape and coloration of adults are distinctive. sanguisuga, is the most commonly encountered species The elongated head narrows toward the front and is in Louisiana, but it is not the only species that occurs in black with slender, six-segmented antennae and a sharp, the state. Of 130 Louisiana specimens in the Louisiana three-segmented beak that folds beneath the head when State Arthropod Museum, 126 are T. sanguisuga, three are the insect is not feeding. The triangular thorax is black Triatoma lecticularia and one is Triatoma gerstaekeri. These with a narrow orange insects are often referred to as “kissing bugs,” a name that is or pinkish-orange used for the entire family Triatominae. margin and a prominent, triangular black Life Cycle scutellum between The eastern conenose and other kissing bugs are the wing bases. The typically associated with mammal burrows, nests or forewings are folded other harborages of small mammals. They require a flat across the abdomen blood meal during each stage of development, thus the when at rest, and each close association with mammals. -
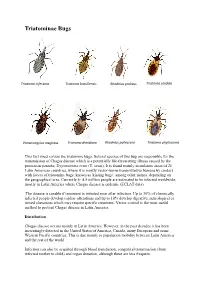
Triatominae Bugs
Triatominae Bugs Triatoma infestans Triatoma brasiliensis Rhodnius prolixus Triatoma sordida Panstrongylus megistus Triatoma dimidiata Rhodnius pallescens Triatoma phyllosoma This fact sheet covers the triatomine bugs. Several species of this bug are responsible for the transmission of Chagas disease which is a potentially life-threatening illness caused by the protozoan parasite, Trypanosoma cruzi (T. cruzi). It is found mainly in endemic areas of 21 Latin American countries, where it is mostly vector-borne transmitted to humans by contact with faeces of triatomine bugs, known as 'kissing bugs', among other names, depending on the geographical area. Currently 4- 4.5 million people are estimated to be infected worldwide, mostly in Latin America where Chagas disease is endemic (ECLAT data). The disease is curable if treatment is initiated soon after infection. Up to 30% of chronically infected people develop cardiac alterations and up to 10% develop digestive, neurological or mixed alterations which may require specific treatment. Vector control is the most useful method to prevent Chagas disease in Latin America. Distribution Chagas disease occurs mainly in Latin America. However, in the past decades it has been increasingly detected in the United States of America, Canada, many European and some Western Pacific countries. This is due mainly to population mobility between Latin America and the rest of the world. Infection can also be acquired through blood transfusion, congenital transmission (from infected mother to child) and organ donation, although these are less frequent. Transmission In Latin America, T. cruzi parasites are mainly transmitted by contact with the faeces of infected blood-sucking triatomine bugs. These bugs, vectors that carry the parasites, typically live in the cracks of poorly-constructed homes in rural or suburban areas. -

Short-Range Responses of the Kissing Bug Triatoma Rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light
insects Article Short-Range Responses of the Kissing Bug Triatoma rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light Andres Indacochea 1, Charlotte C. Gard 2, Immo A. Hansen 3, Jane Pierce 4 and Alvaro Romero 1,* 1 Department of Entomology, Plant Pathology and Weed Science, New Mexico State University, Las Cruces, NM 88003, USA; [email protected] 2 Department of Economics, Applied Statistics, and International Business, New Mexico State University, Las Cruces, NM 88003, USA; [email protected] 3 Department of Biology, New Mexico State University, Las Cruces, NM 88003, USA; [email protected] 4 Department of Entomology, Plant Pathology and Weed Science, New Mexico State University, Artesia, NM 88210, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-575-646-5550 Academic Editors: Changlu Wang and Chow-Yang Lee Received: 20 June 2017; Accepted: 25 August 2017; Published: 29 August 2017 Abstract: The hematophagous bug Triatoma rubida is a species of kissing bug that has been marked as a potential vector for the transmission of Chagas disease in the Southern United States and Northern Mexico. However, information on the distribution of T. rubida in these areas is limited. Vector monitoring is crucial to assess disease risk, so effective trapping systems are required. Kissing bugs utilize extrinsic cues to guide host-seeking, aggregation, and dispersal behaviors. These cues have been recognized as high-value targets for exploitation by trapping systems. a modern video-tracking system was used with a four-port olfactometer system to quantitatively assess the behavioral response of T. rubida to cues of known significance.