Nadcc Tablets for Disinfection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014
Material Safety Data Sheet Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014 SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Sodium Hypochlorite, 10-20% Product code MSDS Number : 10000032 Synonyms : Sodium Hypochlorite - 18, Hypo, Liquid Bleach, Bleach, Hypochlorite, Liquid Chlorine Solution, Javel Water Chemical Family : Hypochlorite Molecular formula : NaOCl Product Use Description : Swimming pool chlorinators, hard surface cleaners, mildecides, Water treatment chemicals, Biocides, bleach solutions and bleach fixer solutions Company Olin Chlor Alkali Products Pioneer Americas, LLC Olin Canada ULC 490 Stuart Road, NE d/b/a Olin Chlor Alkali Products d/b/a Olin Chlor Alkali Products Cleveland, Tennessee 37312 490 Stuart Road, NE 2020 University, Suite 2190 Cleveland, Tennessee 37312 Montreal, Quebec H3A 2A5 Emergency Phone Number : US: 1-800-424-9300 - CHEMTREC CANADA: 1-800-567-7455 SECTION 2. HAZARDS IDENTIFICATION HMIS Classification : Health Hazard: 3 HMIS Flammability: 0 Health Hazard 3 Physical hazards: 2 Flammability 0 Physical hazards 2 NFPA Classification : Health Hazard: 3 Fire Hazard: 0 0 Reactivity Hazard: 1 3 1 Emergency Overview OSHA Hazards : OXIDIZER, UNSTABLE (REACTIVE), CORROSIVE Immediately Dangerous to Life or : Not established for the product. Health Potential Health Effects Primary Routes of Entry : Ingestion, Eyes, Inhalation, Skin Absorption Aggravated Medical Condition : Asthma, Heart disease, Respiratory disorder Inhalation : Inhalation of vapors is irritating to the respiratory system, may cause throat pain and cough. Inhalation of aerosol may cause irritation to the upper respiratory tract. Higher exposure may cause lung edema, circulatory collapse and unconsciousness. 1 / 9 Material Safety Data Sheet Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014 Skin : May cause skin irritation and/or dermatitis. -

Sodium Hypochlorite
SODIUM HYPOCHLORITE What is SODIUM HYPOCHLORITE? Sodium hypochlorite is a liquid with an odor of chlorine. Usually it is clear but some solutions are greenish to yellow in color. Other names for sodium hypochlorite include Clorox , bleach, liquid bleach, sodium oxychloride, Javex, antiformin, showchlon, Chlorox, B-K, Carrel-Dakin Solution, Chloros, Dakin’s Solution, hychlorite, Javelle Water, Mera Industries 2MOm≥B, Milton, modified Dakin’s Solution, Piochlor, and 13% active chlorine. Where can sodium hypochlorite be found and how is it used? Sodium hypochlorite is mainly used as a bleaching agent or disinfectant. A disinfectant kills bacteria that can carry diseases. It is found in consumer and commercial bleaches, cleaning solutions, and disinfectants for drinking water, wastewater and swimming pools. How can people be exposed to sodium hypochlorite? You could be exposed to sodium hypochlorite through: Breathing fumes while using products containing sodium hypochlorite. Drinking water from public drinking water supplies where these chemicals were added to kill bacteria. You could also be exposed by drinking sodium hypochlorite by accident. Touching sodium hypochlorite if gloves are not worn when using products containing it. Eye Contact by splashing sodium hypochlorite during use. People who work where sodium hypochlorite is used to bleach paper and textiles may have slightly higher levels of exposure in all of the above areas. How does sodium hypochlorite work and how can it affect my health? Sodium hypochlorite is a corrosive substance, meaning that it will eat away at materials it contacts. Accidental sodium hypochlorite poisoning can be deadly. Severe injuries can occur to the mouth, throat, esophagus and stomach. -

Sodium Hypochlorite (≤10%) (Lexi-Tox) Brand Names: US
Sodium Hypochlorite (≤10%) (Lexi-Tox) Brand Names: US Anasept; Anasept Antimicrobial [OTC]; Anasept [OTC]; Avenova; Di-Dak-Sol [OTC]; Epicyn; H-Chlor 12 [OTC]; H-Chlor 6 [OTC]; H-Chlor Wound; Hyclodex; HySept [OTC] Pharmacologic Category Disinfectant, Antibacterial (Topical) CAS Registration • 7681-52-9 • 8007-59-8 Use Aqueous solutions of sodium hypochlorite (≤10%) are commonly used to remove laundry stains and as a household cleaner. Household laundry bleach usually contains sodium hypochlorite in a concentration of ~5%. Additional household products that commonly contain sodium hypochlorite include automatic dishwasher rinses, drain cleaners, household disinfectants, mildew removers, shower and bathtub cleaners, and toilet bowl cleaners. In home maintenance, products that contain sodium hypochlorite are used for cleaning concrete and wood (eg, decks), for swimming pool/spa water maintenance, and for retarding mildew growth. Sodium hypochlorite is also used to decontaminate surfaces that have been contaminated by various biological and chemical terrorism agents. Clinical Presentation Dermal: Symptoms ranging from mild irritation and erythema to burns may occur. Household products are less likely to cause significant burns. Children may be more susceptible to adverse reactions. Ingestion: Household products do not commonly cause severe gastrointestinal problems. The ingestion of a small amount of liquid household bleach (3% to 6%) is unlikely to produce significant toxicity and may result in gastrointestinal irritation, nausea, and vomiting, although the severity may increase when a large volume is ingested. Some bleaches may contain sodium hydroxide. Be sure to check the product ingredients carefully. Inhalation: Symptoms following inhalation are concentration-dependent and range from mild mucous membrane irritation to dyspnea and bronchospasm. -

Liquid Bleach Mixing Guidance - Revised
ROY COOPER • Governor MANDY COHEN, MD, MPH • Secretary MARK T. BENTON • Assistant Secretary for Public Health Division of Public Health January 3, 2020 POSITION STATEMENT: Liquid Bleach Mixing Guidance - Revised PURSUANT TO: Rules Governing the Sanitation of Child Care Centers 15A NCAC 18A .2800 SOURCE: Children’s Environmental Health ISSUE: Guidance for compliance with the generic bleach mixing solution for disinfection and for sanitizing as specified in Rule 15A NCAC 18A .2801(7) and (22). QUESTION: Do the mixing specifications as directed in rules .2801(7) and .2801(22) apply to all household liquid chlorine bleach concentrations, such as the 8.25% sodium hypochlorite solution? ANSWER: No DISCUSSION AND RATIONALE: The concentrations of chlorine in readily available household bleach have changed. Currently, one of the most commonly available bleach solutions is 8.25% sodium hypochlorite solution. It should be noted that mixing instructions provided in the child care sanitation rules were based on bleach concentrations of 5.25% and 6% of sodium hypochlorite solution. Because of varying available concentrations for household bleach, it is necessary to provide mixing guidelines and ensure that operators are testing solutions to achieve proper concentrations. RESPONSE/INTERPRETATION: The following is provided as added guidance to assist in compliance with Rules .2801(7) and .2801(22). It is also recommended that child care providers be instructed to read bleach container labels carefully to verify the sodium hypochlorite concentration and to use the following table as a guide for mixing bleach. NC DEPARTMENT OF HEALTH AND HUMAN SERVICES • DIVISION OF PUBLIC HEALTH LOCATION: 5605 SIX FORKS RD, RALEIGH NC 27609 MAILING ADDRESS: 1632 MAIL SERVICE CENTER, RALEIGH NC 27699-1632 www.ncdhhs.gov • TEL: 919-707-5854 • FAX: 919-845-3972 AN EQUAL OPPORTUNITY / AFFIRMATIVE ACTION EMPLOYER ROY COOPER • Governor MANDY COHEN, MD, MPH • Secretary MARK T. -

Disinfection and Waste Management in the ETU
Disinfection and Waste Management in the ETU This lecture is on disinfection and waste management in the ETU. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa Disinfection and Waste Management in the ETU This presentation is current as of December, 2014. This presentation contains materials from Centers for Disease Control and Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). U.S. Department of Health and Human Services Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Identify how and when to use chlorine solution and Identify how and when to use chlorine solution and the the correct strengths for different uses correct strengths for different uses Identify proper ways to dispose of various wastes Describe procedures for disinfection in the ETU ▶ Identify proper ways to dispose of various wastes Describe safe handling, moving, and burial of a corpse ▶ Describe procedures for disinfection in the ETU ▶ Describe safe handling, moving, and burial of a corpse This presentation contains materials from CDC, MSF, and WHO 2 To provide maximum safety in the ETU, it is important for Disinfection in the ETU all healthcare workers to understand the procedures for ETU Considerations disinfection, infection prevention and control, and sanitation Healthcare workers should understand sanitation procedures even if they may not perform all of them described in this lecture. Patients shed large quantities of virus in blood and body fluids ETU disinfection practices are influenced by two properties of Ebola virus has a lipid envelope, making it relatively easy to inactivate the Ebola virus. -
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
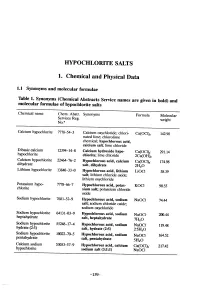
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

SODIUM HYPOCHLORITE, AKA “LIQUID CHLORINE” in Other Words, Bleach by the PHTA Recreational Water Quality Committee
TECH NOTES SODIUM HYPOCHLORITE, AKA “LIQUID CHLORINE” In other words, bleach By the PHTA Recreational Water Quality Committee IN THE SWIMMING pool industry, one gallon of 12.5% sodium hypochlorite a corrosive. As such, a maximum of of the most popularly chosen forms for provides approximately 12.5 ppm of 500 gallons can be stored in a non-fire, sanitizing and superchlorinating water free chlorine per 10,000 gallons of sprinkler-protected room and 1,000 is sodium hypochlorite. Commonly pool water. It takes 10.6 fl. oz of 12.5% gallons in a fire, sprinkler-protected known as “liquid chlorine” or bleach, sodium hypochlorite to get roughly room as maximum exempt quantities. sodium hypochlorite is widely used 1 ppm of free chlorine in 10,000 gallons Quantities beyond this create an “H” in both commercial and residential of pool water. The pH of pool grade Hazardous Occupancy and require swimming pools. Sodium hypochlorite sodium hypochlorite is 13. special fire protection. effectively destroys bacteria and Sodium hypochlorite is Sodium hypochlorite reacts prevents algae in swimming pools. classified as an inorganic sanitizer; in water to create hypochlorous This edition of Tech Notes provides it does not contain cyanuric acid. information on the characteristics, Sodium hypochlorite is a primary effects and proper application of sanitizer because of its ability to SODIUM HYPOCHLORITE: THE sodium hypochlorite. kill microorganisms, oxidize non- living contaminants like ammonia BASIC FACTS WHAT IT IS and swimmers’ waste and provide • Clear yellow liquid with a Sodium hypochlorite (NaOCl), a protective residual in the water. chlorine odor commonly referred to as “liquid Sodium hypochlorite is non-flammable, • A solution containing chlorine” or liquid bleach, is an non-combustible and non-explosive, water, hypochlorite, sodium aqueous solution created by and containers under 1.3 gallons hydroxide and a trace amount mixing chlorine gas in water with aretransported as “Limited Quantities” of sodium chloride concentrations of sodium hydroxide. -

Gel Liquid Bleach
GEL LIQ BLEACH Safety Data Sheet 1. MATERIAL AND SUPPLY COMPANY IDENTIFICATION Product name: GEL LIQUID BLEACH Synonyms Product Code Gel liquid bleach, Chlorfoam, Chloroclean, Bleach foaming chlorinated cleaner 335 Recommended use: Chlorinated detergent. Ideal to disinfect and clean shower tiles, toilets, bathroom floors and work surfaces. Supplier Name CLEAN PLUS CHEMICALS PTY LTD Address 16 George Young Street AUBURN NSW 2144 Telephone 02 9738 7444 Emergency 1800 201 700 Email [email protected] Web Site www.cleanplus.com.au SDS Date 21 JANUARY 2021 Version 1.2 2. HAZARDS IDENTIFICATION This material is hazardous according to the health criteria of Safe Work Australia. Signal Word Danger Hazard Classifications Skin Corrosion/Irritation - Category 1B Serious Eye Damage/Irritation - Category 1 Acute Hazard to the Aquatic Environment - Category 2 Hazard Statements H314 Causes severe skin burns and eye damage. H401 Toxic to aquatic life. Prevention Precautionary Statements P102 Keep out of reach of children. P103 Read label before use. P260 Do not breathe dust, fume, gas, mist, vapours or spray. P264 Wash hands, face and all exposed skin thoroughly after handling. P280 Wear protective clothing, gloves, eye/face protection and suitable respirator. Response Precautionary Statements P101 If medical advice is needed, have product container or label at hand. P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353 IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower. Page 1 of 8 GEL LIQ BLEACH Safety Data Sheet P304+P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

Busting the Myths of CHLORINE DISINFECTION
MANUFACTURING Article Busting the myths of CHLORINE DISINFECTION A successful cleanroom disinfectant needs to meet many criteria, not only in terms of its efficaciousness but also in terms of packaging, ease of use, operator acceptability, etc. Many articles have been written on how to specify and select a cleanroom disinfectant, but this is not the main focus of this article. However, a brief summary of requirements helps when comparing available chlorine chemistries. The ideal cleanroom disinfectant decontamination process is if the Taking as a given good broad spectrum disinfectant has no residue that needs to efficacy including highly resistant be removed or as a minimum is free bacterial spores, the requirements for rinsing. the ideal cleanroom disinfectant are The product will need to have in quite lengthy: a sterile option for grade A excess of a 12-month unopened shelflife and B environments1, non-flammable so and in excess of a three-month in-use it can be used over large areas with no shelflife to be practical to store and use. health and safety concerns, also fast This ideal disinfectant would then need drying with short contact times to reduce to be manufactured to the requirements the time taken for biodecontamination. of cGMP, be notified to the BPR2 and However, in an ideal world, this cannot provided in cleanroom compatible be traded for any problems with either packaging in a variety of formats so it equipment or operators and the wider was suitable for use in all areas of the environment in terms of disposal. cleanroom. It goes without saying that Another requirement shortening the this all needs to be achieved in a cost- effective formulation. -

Oxychem Sodium Hypochlorite Handbook
TABLE OF CONTENTS OxyChem Sodium Hypochlorite Handbook Introduction 2 Foreword Properties 3 This handbook outlines recommended methods for handling, storing, and using sodium hypochlorite. It also Concentration Terminology 5 includes information on the manufacture, physical properties, safety considerations and analytical methods for testing sodium Manufacturing 6 hypochlorite. Additional information and contacts can be found at www.oxychem.com Handling and Storage 9 Safety Handling 11 Unloading Tank Trucks 14 Physical Property Data 16 Methods of Analysis 18 Typical Storage Tank Installation 23 Important: The information presented herein, while not guaranteed, was prepared by technical personnel and is true and accurate to the best of our knowledge. NO WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR A PARTICULAR PURPOSE, OR WARRANTY OR GUARANTY OF ANY OTHER KIND, EXPRESS OR IMPLIED, IS MADE REGARDING PERFORMANCE, SAFETY, SUITABILITY, STABILITY OR OTHERWISE. This information is not intended to be all-inclusive as to the manner and conditions of use, handling, storage, disposal and other factors that may involve other or additional legal, environmental, safety or performance considerations, and Occidental Chemical Corporation assumes no liability whatsoever for the use of or reliance upon this information. While our technical personnel will be happy to respond to questions, safe handling and use of the product remains the responsibility of the customer. No suggestions for use are intended as, and nothing herein shall be construed as, a recommendation to infringe any existing patents or to violate any Federal, State, local or foreign laws. INTRODUCTION This handbook provides information Sodium hypochlorite solutions have In 1798, Tennant of England prepared concerning sodium hypochlorite or attained widespread use in bleaching a solution of calcium hypochlorite by bleach, solutions. -

Sanitizer & Disinfectant Guidance
Sanitizer & Disinfectant Guidance Bleach Used as a Sanitizer or Disinfectant Clorox and other bleach manufacturers are an approved sanitizer and disinfectant. Bleach concentration is now stronger. Find the percentage of sodium hypochlorite (the active ingredient) on bleach bottle. It must be 8.25%. Avoid splashless and scented bleaches. Bleach to Water Mixing Instructions: Mix bleach with cool water to make solution using the chart the below as a guide. Amount of Bleach for Amount of Bleach for Amount of Water Sanitizer (50-200 ppm) Disinfectant (> 2400 ppm) 1 Gallon (128 ounces) 1 to 2 Teaspoons 8 Tablespoons or 1/2 cup (4 oz.) 1 Quart (32 ounces) ¼ to ½ Teaspoon 2 Tablespoons (1 oz.) 1 Pint (16 ounces) ⅛ to ¼ Teaspoon 1 Tablespoon (.05 oz.) **Always use Chlorine test Concentration: 50-200 ppm Concentration: > 2400 ppm strips to ensure proper concentration. Hydrion™ Chlorine Test Strips or similar brands are available for purchase online. Disinfect bathrooms, diaper changing Sanitize toys, food preparation tables, toilet seats, surfaces contami- Clean surfaces first to re- surfaces, tables, mats, door nated with high hazard fluids (vomit, move visible soil, dirt and knobs, highchairs contamination before stool, urine, blood) using bleach solution. Contact time: 2 minutes; air Contact time: 5 minutes; air dry then dry, no rinse step. rinse with water. For safety reasons, add bleach to water, not water to bleach. Do not mix liquid bleach with other cleaning products or ammonia, which may release hazardous gases into the air. Other Sanitizers & Disinfectants: How to Know if they are Approved? Manufacturers of sanitizers with different active ingredients, such as quaternary ammonium, also make approved products. -

Cholera Outbreak Guidelines: Preparedness, Prevention and Control
CHOLERA OUTBREAK GUIDELINES PREPAREDNESS, PREVENTION AND CONTROL Elizabeth Lamond and Jesee Kinyanjui June 2012 Table of Contents PREFACE ......................................................................................................................... 4 ACKNOWLEDGEMENTS ...................................................................................................... 4 ABBREVIATIONS ............................................................................................................... 5 1. INTRODUCTION .............................................................................................................. 6 1.1 Overview ............................................................................................................... 6 1.2 About cholera and its transmission routes ............................................................. 6 1.3 Risk factors ............................................................................................................ 7 2. NON-ENDEMIC COUNTRY (NEW) OUTBREAKS................................................................. 10 2.1 Responding to non-endemic outbreaks ............................................................... 10 3. PRE-OUTBREAK PHASE ............................................................................................... 12 3.1 Cholera preparedness and action plans (endemic countries) .............................. 12 3.2 Key components of a good cholera preparedness plan ....................................... 12 4. TRANSITION FROM PREPAREDNESS