J:\Med Mgmt Guide\MMG FA268.0.121\Draft 3
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Mechanism of Action of Sodium Hypochlorite ISSN 0103-6440113
Braz Dent J (2002) 13(2): 113-117 Mechanism of action of sodium hypochlorite ISSN 0103-6440113 Mechanism of Action of Sodium Hypochlorite Carlos ESTRELA1 Cyntia R.A. ESTRELA1 Eduardo Luis BARBIN2 Júlio César E. SPANÓ2 Melissa A. MARCHESAN2 Jesus D. PÉCORA2 1Faculty of Dentistry, Federal University of Goiás, Goiânia, GO, Brazil 2Faculty of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil The choice of an irrigating solution for use in infected root canals requires previous knowledge of the microorganisms responsible for the infectious process as well as the properties of different irrigating solutions. Complex internal anatomy, host defenses and microorganism virulence are important factors in the treatment of teeth with asymptomatic apical periodontitis. Irrigating solutions must have expressive antimicrobial action and tissue dissolution capacity. Sodium hypochlorite is the most used irrigating solution in endodontics, because its mechanism of action causes biosynthetic alterations in cellular metabolism and phospholipid destruction, formation of chloramines that interfere in cellular metabolism, oxidative action with irreversible enzymatic inactivation in bacteria, and lipid and fatty acid degradation. The aim of this work is to discuss the mechanism of action of sodium hypochlorite based on its antimicrobial and physico-chemical properties. Key Words: sodium hypochlorite, irrigating solution, intracanal dressing. INTRODUCTION microbial agent to the infected site, adequate concen- tration of the agent, -

Disinfection Session Objectives
Disinfection Session Objectives • To introduce the principal disinfectants that may be used and highlight key advantages and disadvantages of each • To emphasise the use of chlorination for routine disinfection. • To describe the process of chlorination and discuss the concepts of breakpoint chlorination, chlorine demand and outline basic chlorine chemistry. • To discuss the types of chlorine available and how these may be used for routine disinfection. WHO SEMINAR PACK FOR DRINKING-WATER QUALITY Disinfection Introduction All water supplies should be disinfected. This is aimed both at inactivating remaining bacteria before distribution and providing a residual disinfectant to inactivate bacteria introduced by any subsequent ingress of contaminated water during storage or distribution. At present, the principal disinfectant used worldwide is chlorine, although alternatives are being increasingly investigated and process such as ozonation are becoming more common. Chlorine is generally the disinfectant of choice as it is reasonably efficient, cheap and easy to handle. In all but the smallest water treatment plants, chlorine is added to water as either in aqueous solution (calcium hypochlorite or sodium hypochlorite) or chlorine gas. Smaller supplies may use tablets of hypochlorite. Other disinfectants include ozone, ultraviolet light and iodine. These all have disadvantages. UV is not a particularly effective disinfectant and it is difficult to expose water for sufficient time for disinfection to be effective. Neither ozone or UV provide a residual disinfectant and therefore offer no protection against recontamination in distribution. To overcome this, in some water supplies booster ozonation stations are set up along the distribution network. Both iodine and ozone are carcinogenic. There are also significant health and safety concerns, for operators, regarding the generation and application of ozone and chlorine (especially in the gaseous form). -

Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014
Material Safety Data Sheet Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014 SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Sodium Hypochlorite, 10-20% Product code MSDS Number : 10000032 Synonyms : Sodium Hypochlorite - 18, Hypo, Liquid Bleach, Bleach, Hypochlorite, Liquid Chlorine Solution, Javel Water Chemical Family : Hypochlorite Molecular formula : NaOCl Product Use Description : Swimming pool chlorinators, hard surface cleaners, mildecides, Water treatment chemicals, Biocides, bleach solutions and bleach fixer solutions Company Olin Chlor Alkali Products Pioneer Americas, LLC Olin Canada ULC 490 Stuart Road, NE d/b/a Olin Chlor Alkali Products d/b/a Olin Chlor Alkali Products Cleveland, Tennessee 37312 490 Stuart Road, NE 2020 University, Suite 2190 Cleveland, Tennessee 37312 Montreal, Quebec H3A 2A5 Emergency Phone Number : US: 1-800-424-9300 - CHEMTREC CANADA: 1-800-567-7455 SECTION 2. HAZARDS IDENTIFICATION HMIS Classification : Health Hazard: 3 HMIS Flammability: 0 Health Hazard 3 Physical hazards: 2 Flammability 0 Physical hazards 2 NFPA Classification : Health Hazard: 3 Fire Hazard: 0 0 Reactivity Hazard: 1 3 1 Emergency Overview OSHA Hazards : OXIDIZER, UNSTABLE (REACTIVE), CORROSIVE Immediately Dangerous to Life or : Not established for the product. Health Potential Health Effects Primary Routes of Entry : Ingestion, Eyes, Inhalation, Skin Absorption Aggravated Medical Condition : Asthma, Heart disease, Respiratory disorder Inhalation : Inhalation of vapors is irritating to the respiratory system, may cause throat pain and cough. Inhalation of aerosol may cause irritation to the upper respiratory tract. Higher exposure may cause lung edema, circulatory collapse and unconsciousness. 1 / 9 Material Safety Data Sheet Sodium Hypochlorite, 10-20% Version 7 Revision Date 02/20/2014 Print Date 02/20/2014 Skin : May cause skin irritation and/or dermatitis. -

Sodium Hypochlorite
SODIUM HYPOCHLORITE What is SODIUM HYPOCHLORITE? Sodium hypochlorite is a liquid with an odor of chlorine. Usually it is clear but some solutions are greenish to yellow in color. Other names for sodium hypochlorite include Clorox , bleach, liquid bleach, sodium oxychloride, Javex, antiformin, showchlon, Chlorox, B-K, Carrel-Dakin Solution, Chloros, Dakin’s Solution, hychlorite, Javelle Water, Mera Industries 2MOm≥B, Milton, modified Dakin’s Solution, Piochlor, and 13% active chlorine. Where can sodium hypochlorite be found and how is it used? Sodium hypochlorite is mainly used as a bleaching agent or disinfectant. A disinfectant kills bacteria that can carry diseases. It is found in consumer and commercial bleaches, cleaning solutions, and disinfectants for drinking water, wastewater and swimming pools. How can people be exposed to sodium hypochlorite? You could be exposed to sodium hypochlorite through: Breathing fumes while using products containing sodium hypochlorite. Drinking water from public drinking water supplies where these chemicals were added to kill bacteria. You could also be exposed by drinking sodium hypochlorite by accident. Touching sodium hypochlorite if gloves are not worn when using products containing it. Eye Contact by splashing sodium hypochlorite during use. People who work where sodium hypochlorite is used to bleach paper and textiles may have slightly higher levels of exposure in all of the above areas. How does sodium hypochlorite work and how can it affect my health? Sodium hypochlorite is a corrosive substance, meaning that it will eat away at materials it contacts. Accidental sodium hypochlorite poisoning can be deadly. Severe injuries can occur to the mouth, throat, esophagus and stomach. -

Sodium Hypochlorite (≤10%) (Lexi-Tox) Brand Names: US
Sodium Hypochlorite (≤10%) (Lexi-Tox) Brand Names: US Anasept; Anasept Antimicrobial [OTC]; Anasept [OTC]; Avenova; Di-Dak-Sol [OTC]; Epicyn; H-Chlor 12 [OTC]; H-Chlor 6 [OTC]; H-Chlor Wound; Hyclodex; HySept [OTC] Pharmacologic Category Disinfectant, Antibacterial (Topical) CAS Registration • 7681-52-9 • 8007-59-8 Use Aqueous solutions of sodium hypochlorite (≤10%) are commonly used to remove laundry stains and as a household cleaner. Household laundry bleach usually contains sodium hypochlorite in a concentration of ~5%. Additional household products that commonly contain sodium hypochlorite include automatic dishwasher rinses, drain cleaners, household disinfectants, mildew removers, shower and bathtub cleaners, and toilet bowl cleaners. In home maintenance, products that contain sodium hypochlorite are used for cleaning concrete and wood (eg, decks), for swimming pool/spa water maintenance, and for retarding mildew growth. Sodium hypochlorite is also used to decontaminate surfaces that have been contaminated by various biological and chemical terrorism agents. Clinical Presentation Dermal: Symptoms ranging from mild irritation and erythema to burns may occur. Household products are less likely to cause significant burns. Children may be more susceptible to adverse reactions. Ingestion: Household products do not commonly cause severe gastrointestinal problems. The ingestion of a small amount of liquid household bleach (3% to 6%) is unlikely to produce significant toxicity and may result in gastrointestinal irritation, nausea, and vomiting, although the severity may increase when a large volume is ingested. Some bleaches may contain sodium hydroxide. Be sure to check the product ingredients carefully. Inhalation: Symptoms following inhalation are concentration-dependent and range from mild mucous membrane irritation to dyspnea and bronchospasm. -

Liquid Bleach Mixing Guidance - Revised
ROY COOPER • Governor MANDY COHEN, MD, MPH • Secretary MARK T. BENTON • Assistant Secretary for Public Health Division of Public Health January 3, 2020 POSITION STATEMENT: Liquid Bleach Mixing Guidance - Revised PURSUANT TO: Rules Governing the Sanitation of Child Care Centers 15A NCAC 18A .2800 SOURCE: Children’s Environmental Health ISSUE: Guidance for compliance with the generic bleach mixing solution for disinfection and for sanitizing as specified in Rule 15A NCAC 18A .2801(7) and (22). QUESTION: Do the mixing specifications as directed in rules .2801(7) and .2801(22) apply to all household liquid chlorine bleach concentrations, such as the 8.25% sodium hypochlorite solution? ANSWER: No DISCUSSION AND RATIONALE: The concentrations of chlorine in readily available household bleach have changed. Currently, one of the most commonly available bleach solutions is 8.25% sodium hypochlorite solution. It should be noted that mixing instructions provided in the child care sanitation rules were based on bleach concentrations of 5.25% and 6% of sodium hypochlorite solution. Because of varying available concentrations for household bleach, it is necessary to provide mixing guidelines and ensure that operators are testing solutions to achieve proper concentrations. RESPONSE/INTERPRETATION: The following is provided as added guidance to assist in compliance with Rules .2801(7) and .2801(22). It is also recommended that child care providers be instructed to read bleach container labels carefully to verify the sodium hypochlorite concentration and to use the following table as a guide for mixing bleach. NC DEPARTMENT OF HEALTH AND HUMAN SERVICES • DIVISION OF PUBLIC HEALTH LOCATION: 5605 SIX FORKS RD, RALEIGH NC 27609 MAILING ADDRESS: 1632 MAIL SERVICE CENTER, RALEIGH NC 27699-1632 www.ncdhhs.gov • TEL: 919-707-5854 • FAX: 919-845-3972 AN EQUAL OPPORTUNITY / AFFIRMATIVE ACTION EMPLOYER ROY COOPER • Governor MANDY COHEN, MD, MPH • Secretary MARK T. -

Nitrogen, Ammonia, Colorimetry, Salicylate-Hypochlorite, Automated-Segmented Flow
Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/L as N): 00608 Nitrogen, ammonia, total-in-bottom-material, I-6522-90 (mg/L as N): 00611 1. Application 1.1 This method is used to analyze samples of surface, domestic, and industrial water, and brines containing from 0.01 to 1.5 mg/L of ammonia-nitrogen. Concentrations greater than 1.50 mg/L must be diluted. This modified method was implemented in the National Water Quality Laboratory in March 1988. 1.2 This method also is used to determine concentrations of ammonia-nitrogen in samples of bottom material containing at least 0.2 mg/kg NH3-N. Prepared sample solutions containing more than 1.5 mg/L NH3-N need to be diluted. 1.3 Sodium ion is a good replacement for ammonium ion in the slow-exchange positions of soil minerals (Jackson, 1958). Bottom material is treated with an acidified sodium chloride solution, and the resulting mixture is allowed to settle and then decanted to obtain a clear supernatant solution for analysis. 2. Summary of method Ammonia reacts with salicylate and hypochlorite ions in the presence of ferricyanide ions to form the salicylic acid analog of indophenol blue (Reardon and others, 1966; Patton and Crouch, 1977; Harfmann and Crouch, 1989). The resulting color is directly proportional to the concentration of ammonia present. 3. Interferences 3.1 Sulfide interferes. Bromide and nitrite can interfere. Calcium and magnesium in highly alkaline waters (pH greater than 13.6) can exceed the ability of the tartrate to complex both ions. -

COVID-19 Environmental Cleaning and Disinfectants for Clinic
Coronavirus COVID-19 BC Centre for Disease Control | BC Ministry of Health Environmental Cleaning and Disinfectants for Health-Care and Clinic Settings Cleaning: the physical removal of visible soiling (e.g., dust, soil, blood, mucus). Cleaning removes, rather than kills, viruses and bacteria. It is done with water, detergents, and steady friction from cleaning cloth. Disinfection: the killing of viruses and bacteria. A disinfectant is only applied to objects; never on the human body. All visibly soiled surfaces should be cleaned before disinfection. Environmental cleaning for the COVID-19 virus is the same as for other common viruses. Cleaning products and disinfectants that are regularly used in hospitals and health-care settings are strong enough to deactivate coronaviruses and prevent their spread. Cleaning of visibly soiled surfaces followed by disinfection is recommended for the prevention of COVID-19 and other viral respiratory illnesses. Suggested cleaning and disinfecting frequencies for health-care and clinic settings: Type of surface Frequency 1. Shared equipment IN BETWEEN PATIENTS otoscopes, baby scales, tables and exam beds 2. Frequently-touched surfaces Examples: medical equipment, door knobs, light AT LEAST TWICE A DAY switches, telephones, keyboards, mice, pens, charts, cell phones, toys, bathrooms 3. General cleaning of procedure / exam rooms AT LEAST ONCE A DAY For electronic equipment please comply with manufacturer’s instructions in order to meet warranty requirements 06.02.21 Coronavirus COVID-19 BC Centre for Disease Control | BC Ministry of Health Environmental Cleaning and Disinfectants for Health-Care and Clinic Settings The list of common disinfectants below is provided as a guide to choosing products. -

Disinfection and Waste Management in the ETU
Disinfection and Waste Management in the ETU This lecture is on disinfection and waste management in the ETU. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa Disinfection and Waste Management in the ETU This presentation is current as of December, 2014. This presentation contains materials from Centers for Disease Control and Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). U.S. Department of Health and Human Services Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Identify how and when to use chlorine solution and Identify how and when to use chlorine solution and the the correct strengths for different uses correct strengths for different uses Identify proper ways to dispose of various wastes Describe procedures for disinfection in the ETU ▶ Identify proper ways to dispose of various wastes Describe safe handling, moving, and burial of a corpse ▶ Describe procedures for disinfection in the ETU ▶ Describe safe handling, moving, and burial of a corpse This presentation contains materials from CDC, MSF, and WHO 2 To provide maximum safety in the ETU, it is important for Disinfection in the ETU all healthcare workers to understand the procedures for ETU Considerations disinfection, infection prevention and control, and sanitation Healthcare workers should understand sanitation procedures even if they may not perform all of them described in this lecture. Patients shed large quantities of virus in blood and body fluids ETU disinfection practices are influenced by two properties of Ebola virus has a lipid envelope, making it relatively easy to inactivate the Ebola virus. -

Perchlorate in Sodium Hypochlorite
emerging issues BY PETER GREINER, CLIF MCLELLAN, DALE BENNETT, AND ANGIE EWING Occurrence of perchlorate in sodium hypochlorite RESPONDING TO THE DETECTION erchlorate is both a synthetic and a naturally occurring chemical. Most of the perchlorate that is manufactured in the United States OF PERCHLORATE IN SODIUM is used as the primary ingredient of solid rocket propellant. Wastes HYPOCHLORITE BY THE from the manufacture and improper disposal of perchlorate-con- taining chemicals are increasingly being discovered in soil and water MASSACHUSETTS DEPARTMENT P (USEPA, 2007). OF ENVIRONMENTAL An additional source of perchlorate in drinking water has been found to occur through the use of sodium hypochlorite. The Massachusetts Department PROTECTION, of Environmental Protection (MDEP) has reported that significant levels of NSF INTERNATIONAL SURVEYED perchlorate can be detected in sodium hypochlorite samples that have aged for a few weeks (MDEP, 2005). Sodium hypochlorite as delivered to one utility FOR THE CONTAMINANT had a perchlorate concentration of 0.2 µg/L in the product, but the level of IN DRINKING WATER TREATMENT perchlorate rose to 6,750 µg/L after the product had aged for 26 days. CHEMICALS FROM PRODUCTION INVESTIGATION OF WATER TREATMENT CHEMICALS BEGAN IN 2005 FACILITIES ACROSS In 2005 NSF International began analyzing samples of drinking water treat- ment chemicals for the contaminant perchlorate. These samples were collected THE UNITED STATES as part of the annual testing requirement to support NSF certification of the AND CANADA. treatment chemical to NSF/American National Standards Institute Standard 60: Drinking Water Treatment Chemicals—Health Effects (NSF/ANSI, 2005). Samples collected included not only sodium hypochlorite but other types of chemicals as well. -
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
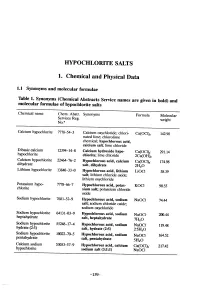
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

SODIUM HYPOCHLORITE, AKA “LIQUID CHLORINE” in Other Words, Bleach by the PHTA Recreational Water Quality Committee
TECH NOTES SODIUM HYPOCHLORITE, AKA “LIQUID CHLORINE” In other words, bleach By the PHTA Recreational Water Quality Committee IN THE SWIMMING pool industry, one gallon of 12.5% sodium hypochlorite a corrosive. As such, a maximum of of the most popularly chosen forms for provides approximately 12.5 ppm of 500 gallons can be stored in a non-fire, sanitizing and superchlorinating water free chlorine per 10,000 gallons of sprinkler-protected room and 1,000 is sodium hypochlorite. Commonly pool water. It takes 10.6 fl. oz of 12.5% gallons in a fire, sprinkler-protected known as “liquid chlorine” or bleach, sodium hypochlorite to get roughly room as maximum exempt quantities. sodium hypochlorite is widely used 1 ppm of free chlorine in 10,000 gallons Quantities beyond this create an “H” in both commercial and residential of pool water. The pH of pool grade Hazardous Occupancy and require swimming pools. Sodium hypochlorite sodium hypochlorite is 13. special fire protection. effectively destroys bacteria and Sodium hypochlorite is Sodium hypochlorite reacts prevents algae in swimming pools. classified as an inorganic sanitizer; in water to create hypochlorous This edition of Tech Notes provides it does not contain cyanuric acid. information on the characteristics, Sodium hypochlorite is a primary effects and proper application of sanitizer because of its ability to SODIUM HYPOCHLORITE: THE sodium hypochlorite. kill microorganisms, oxidize non- living contaminants like ammonia BASIC FACTS WHAT IT IS and swimmers’ waste and provide • Clear yellow liquid with a Sodium hypochlorite (NaOCl), a protective residual in the water. chlorine odor commonly referred to as “liquid Sodium hypochlorite is non-flammable, • A solution containing chlorine” or liquid bleach, is an non-combustible and non-explosive, water, hypochlorite, sodium aqueous solution created by and containers under 1.3 gallons hydroxide and a trace amount mixing chlorine gas in water with aretransported as “Limited Quantities” of sodium chloride concentrations of sodium hydroxide.