Habitat Associations and Natural History of the Tasmanian "Snow Skinks" (Niveoscincus Spp.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2014 Conservation Outlook Assessment (Archived)
IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Tasmanian Wilderness - 2014 Conservation Outlook Assessment (archived) IUCN Conservation Outlook Assessment 2014 (archived) Finalised on 07 November 2014 Please note: this is an archived Conservation Outlook Assessment for Tasmanian Wilderness. To access the most up-to-date Conservation Outlook Assessment for this site, please visit https://www.worldheritageoutlook.iucn.org. Tasmanian Wilderness SITE INFORMATION Country: Australia Inscribed in: 1989 Criteria: (iii) (iv) (vi) (vii) (viii) (ix) (x) Site description: In a region that has been subjected to severe glaciation, these parks and reserves, with their steep gorges, covering an area of over 1 million ha, constitute one of the last expanses of temperate rainforest in the world. Remains found in limestone caves attest to the human occupation of the area for more than 20,000 years. © UNESCO IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Tasmanian Wilderness - 2014 Conservation Outlook Assessment (archived) SUMMARY 2014 Conservation Outlook Good with some concerns Competing land-use claims along the boundaries of the Tasmanian Wilderness has been a contentious issue ever since the inscription of the property in 1982 and its further extension in 1989. The recent boundary extensions of 2010, 2012 and 2013 have contributed to the Outstanding Universal Value of the site and improved the scope for effective management of the property. Despite considerable management efforts, a high number of threats face both the initially inscribed property and areas to which it was extended. The biggest issues arise from inadequate resourcing of scientific research into WH values and monitoring; increasing pressures to allow intrusive commercial tourism which could impact heavily on key sites and WH values; protection and management of areas which have been recently added to the property. -

Great Australian Bight BP Oil Drilling Project
Submission to Senate Inquiry: Great Australian Bight BP Oil Drilling Project: Potential Impacts on Matters of National Environmental Significance within Modelled Oil Spill Impact Areas (Summer and Winter 2A Model Scenarios) Prepared by Dr David Ellis (BSc Hons PhD; Ecologist, Environmental Consultant and Founder at Stepping Stones Ecological Services) March 27, 2016 Table of Contents Table of Contents ..................................................................................................... 2 Executive Summary ................................................................................................ 4 Summer Oil Spill Scenario Key Findings ................................................................. 5 Winter Oil Spill Scenario Key Findings ................................................................... 7 Threatened Species Conservation Status Summary ........................................... 8 International Migratory Bird Agreements ............................................................. 8 Introduction ............................................................................................................ 11 Methods .................................................................................................................... 12 Protected Matters Search Tool Database Search and Criteria for Oil-Spill Model Selection ............................................................................................................. 12 Criteria for Inclusion/Exclusion of Threatened, Migratory and Marine -

Overview of Tasmania's Offshore Islands and Their Role in Nature
Papers and Proceedings of the Royal Society of Tasmania, Volume 154, 2020 83 OVERVIEW OF TASMANIA’S OFFSHORE ISLANDS AND THEIR ROLE IN NATURE CONSERVATION by Sally L. Bryant and Stephen Harris (with one text-figure, two tables, eight plates and two appendices) Bryant, S.L. & Harris, S. 2020 (9:xii): Overview of Tasmania’s offshore islands and their role in nature conservation.Papers and Proceedings of the Royal Society of Tasmania 154: 83–106. https://doi.org/10.26749/rstpp.154.83 ISSN: 0080–4703. Tasmanian Land Conservancy, PO Box 2112, Lower Sandy Bay, Tasmania 7005, Australia (SLB*); Department of Archaeology and Natural History, College of Asia and the Pacific, Australian National University, Canberra, ACT 2601 (SH). *Author for correspondence: Email: [email protected] Since the 1970s, knowledge of Tasmania’s offshore islands has expanded greatly due to an increase in systematic and regional surveys, the continuation of several long-term monitoring programs and the improved delivery of pest management and translocation programs. However, many islands remain data-poor especially for invertebrate fauna, and non-vascular flora, and information sources are dispersed across numerous platforms. While more than 90% of Tasmania’s offshore islands are statutory reserves, many are impacted by a range of disturbances, particularly invasive species with no decision-making framework in place to prioritise their management. This paper synthesises the significant contribution offshore islands make to Tasmania’s land-based natural assets and identifies gaps and deficiencies hampering their protection. A continuing focus on detailed gap-filling surveys aided by partnership restoration programs and collaborative national forums must be strengthened if we are to capitalise on the conservation benefits islands provide in the face of rapidly changing environmental conditions and pressure for future use. -

Nowhere Else on Earth
Nowhere Else on Earth: Tasmania’s Marine Natural Values Environment Tasmania is a not-for-profit conservation council dedicated to the protection, conservation and rehabilitation of Tasmania’s natural environment. Australia’s youngest conservation council, Environment Tasmania was established in 2006 and is a peak body representing over 20 Tasmanian environment groups. Prepared for Environment Tasmania by Dr Karen Parsons of Aquenal Pty Ltd. Report citation: Parsons, K. E. (2011) Nowhere Else on Earth: Tasmania’s Marine Natural Values. Report for Environment Tasmania. Aquenal, Tasmania. ISBN: 978-0-646-56647-4 Graphic Design: onetonnegraphic www.onetonnegraphic.com.au Online: Visit the Environment Tasmania website at: www.et.org.au or Ocean Planet online at www.oceanplanet.org.au Partners: With thanks to the The Wilderness Society Inc for their financial support through the WildCountry Small Grants Program, and to NRM North and NRM South. Front Cover: Gorgonian fan with diver (Photograph: © Geoff Rollins). 2 Waterfall Bay cave (Photograph: © Jon Bryan). Acknowledgements The following people are thanked for their assistance The majority of the photographs in the report were with the compilation of this report: Neville Barrett of the generously provided by Graham Edgar, while the following Institute for Marine and Antarctic Studies (IMAS) at the additional contributors are also acknowledged: Neville University of Tasmania for providing information on key Barrett, Jane Elek, Sue Wragge, Chris Black, Jon Bryan, features of Tasmania’s marine -
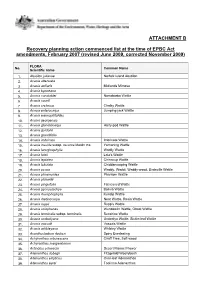
Recovery Planning Action Commenced List (Revised June 2009)
ATTACHMENT B Recovery planning action commenced list at the time of EPBC Act amendments, February 2007 (revised June 2009, corrected November 2009) FLORA No. Common Name Scientific name 1. Abutilon julianae Norfolk Island Abutilon 2. Acacia attenuata 3. Acacia axillaris Midlands Mimosa 4. Acacia bynoeana 5. Acacia constablei Narrabarba Wattle 6. Acacia courtii 7. Acacia cretacea Chalky Wattle 8. Acacia enterocarpa Jumping-jack Wattle 9. Acacia eremophiloides 10. Acacia georgensis 11. Acacia glandulicarpa Hairy-pod Wattle 12. Acacia gordonii 13. Acacia grandifolia 14. Acacia imbricata Imbricate Wattle 15. Acacia insolita subsp. recurva Maslin ms. Yornaning Wattle 16. Acacia lanuginophylla Woolly Wattle 17. Acacia latzii Latz's Wattle 18. Acacia leptalea Chinocup Wattle 19. Acacia lobulata Chiddarcooping Wattle 20. Acacia peuce Waddy, Waddi, Waddy-wood, Birdsville Wattle 21. Acacia phasmoides Phantom Wattle 22. Acacia pickardii 23. Acacia pinguifolia Fat-leaved Wattle 24. Acacia pycnostachya Bolivia Wattle 25. Acacia rhamphophylla Kundip Wattle 26. Acacia rhetinocarpa Neat Wattle, Resin Wattle 27. Acacia ruppii Rupp's Wattle 28. Acacia sciophanes Wundowlin Wattle, Ghost Wattle 29. Acacia terminalis subsp. terminalis Sunshine Wattle 30. Acacia undoolyana Undoolya Wattle, Sickle-leaf Wattle 31. Acacia vassalii Vassals Wattle 32. Acacia whibleyana Whibley Wattle 33. Acanthocladium dockeri Spiny Everlasting 34. Achyranthes arborescens Chaff Tree, Soft-wood 35. Achyranthes margaretarum 36. Actinotus schwarzii Desert Flannel Flower 37. Adenanthos dobagii Fitzgerald Woollybush 38. Adenanthos ellipticus Oval-leaf Adenanthos 39. Adenanthos eyrei Toolinna Adenanthos FLORA No. Common Name Scientific name 40. Adenanthos pungens subsp. effusus Sprawling Spiky Adenanthos 41. Agrostis adamsonii Adamson's Blown-grass = Lachnagrostis adamsonii 42. Allocasuarina robusta Mount Compass Oak-bush 43. -

A. EPBC Listed Species
The following lists may be useful to help you recognise significant species, communities and habitats which may be found in your project area. A. EPBC listed species EPBC species – flora (vascular and non-vascular) EPBC species – fauna alpine leafy liverwort Pseudocephalozia Antarctic Tern Sterna vittata bethunei paludicola apsley heath Epacris apsleyensis Arthurs Paragalaxias Paragalaxias mesotes basalt guineaflower Hibbertia basaltica Australasian Bittern Botaurus poiciloptilus soft peppercress Lepidium hyssopifolium Australian Grayling Prototroctes maraena (basalt peppercress) bearded heath Epacris barbata Black-browed Albatross Thalassarche melanophris (freycinet heath) blacktip spider-orchid Caladenia anthracina Blue Petrel Halobaena caerulea blue wallabygrass Rytidosperma popinensis Blue Whale Balaenoptera musculus bordered heath Epacris limbata Broad-toothed Stag Beetle Lissotes latidens Wielangta Stag Beetle hairy cliff eyebright Euphrasia phragmostoma Clarence Galaxias Galaxias johnstoni (buftons eyebright) chestnut leek-orchid Prasophyllum castaneum Derwent River Seastar Marginaster littoralis clover glycine Glycine latrobeana Eastern-barred Bandicoot Perameles gunnii gunnii (Tasmania) clubmoss bushpea Stonesiella selaginoides Sternula nereis sub sp. Australian Fairy Tern nereis claspleaf heath Epacris acuminata Southern Fairy Prion Pachyptila turtur subantarctica crowded leek-orchid Prasophyllum crebriflorum Fin Whale Balaenoptera physalus curly sedge Carex tasmanica Forty-spotted Pardalote Pardalotus quadragintus grassland -

Appendix 7-2 Protected Matters Search Tool (PMST) Report for the Risk EMBA
Environment plan Appendix 7-2 Protected matters search tool (PMST) report for the Risk EMBA Stromlo-1 exploration drilling program Equinor Australia B.V. Level 15 123 St Georges Terrace PERTH WA 6000 Australia February 2019 www.equinor.com.au EPBC Act Protected Matters Report This report provides general guidance on matters of national environmental significance and other matters protected by the EPBC Act in the area you have selected. Information on the coverage of this report and qualifications on data supporting this report are contained in the caveat at the end of the report. Information is available about Environment Assessments and the EPBC Act including significance guidelines, forms and application process details. Report created: 13/09/18 14:02:20 Summary Details Matters of NES Other Matters Protected by the EPBC Act Extra Information Caveat Acknowledgements This map may contain data which are ©Commonwealth of Australia (Geoscience Australia), ©PSMA 2010 Coordinates Buffer: 1.0Km Summary Matters of National Environmental Significance This part of the report summarises the matters of national environmental significance that may occur in, or may relate to, the area you nominated. Further information is available in the detail part of the report, which can be accessed by scrolling or following the links below. If you are proposing to undertake an activity that may have a significant impact on one or more matters of national environmental significance then you should consider the Administrative Guidelines on Significance. World Heritage Properties: 11 National Heritage Places: 13 Wetlands of International Importance: 13 Great Barrier Reef Marine Park: None Commonwealth Marine Area: 2 Listed Threatened Ecological Communities: 14 Listed Threatened Species: 311 Listed Migratory Species: 97 Other Matters Protected by the EPBC Act This part of the report summarises other matters protected under the Act that may relate to the area you nominated. -

Cotinga 33 Contents
Cotinga 33 Contents News & Reviews 2 Advertising Information 136 New records of Sulphur-breasted Parakeet Aratinga 3 Club News maculata in Pará and Amapá states, Brazil Thiago Vernaschi Vieira da Costa, Christian Borges Andretti, Fábio Olmos & José 120 Short Communications Fernando Pacheco 120 Nuevos registros de Columbina minuta, Pionus senilis y 137 Marsh Seedeater Sporophila palustris and Tawny-bellied Basileuterus culicivorus en el estado de Yucatán, México Seedeater S. hypoxantha recorded in Tocantins state, Brazil Juan Chablé-Santos, Celia Sélem-Salas & Silvia Hernández- Fábio Olmos & José Fernando Pacheco Betancourt 138 First records of Blue-billed Black Tyrant Knipolegus 121 La Tangara Aliamarilla Thraupis abbas en Costa Rica, cyanirostris for Goiás, Brazil Iubatã Paula de Faria, Sandro historia y dos nuevos registros Andrés Zuñiga & Barata Berg, Tarcísio Lyra dos Santos Abreu, Ana Paula Diniz Luis Sandoval Nakamura & Pedro Diniz 122 Deadly intra-specific aggression in Collared Aracari 140 New data on the breeding biology of Gilt-edged Tanager Pteroglossus torquatus Jeffrey D. Ritterson & Adam C. Stein Tangara cyanoventris Carlos Otávio Araujo Gussoni & Pedro 123 First record of Sungrebe Heliornis fulica on Bonaire, Ferreira Develey Netherlands Antilles Peter J. Rozemeijer 140 Primeiro registro do criticamente ameaçado pica-pau-do- 124 The nest and eggs of Yellow-throated Bush Tanager parnaíba Celeus obrieni no Estado do Mato Grosso (Brasil) Chlorospingus flavigularis Harold F. Greeney, Bryan Suson, e comentários sobre distribuição geográfica e conservação Rudy A. Gelis, Ben Freeman & Eliot T. Miller Túlio Dornas, Gabriel Augusto Leite, Renato Torres Pinheiro & 125 The nest and eggs of Blue-and-black Tanager Tangara Marco Aurélio Crozariol vassorii Harold F. -

2020 Conservation Outlook Assessment
IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Tasmanian Wilderness - 2020 Conservation Outlook Assessment Tasmanian Wilderness 2020 Conservation Outlook Assessment SITE INFORMATION Country: Australia Inscribed in: 1989 Criteria: (iii) (iv) (vi) (vii) (viii) (ix) (x) In a region that has been subjected to severe glaciation, these parks and reserves, with their steep gorges, covering an area of over 1 million ha, constitute one of the last expanses of temperate rainforest in the world. Remains found in limestone caves attest to the human occupation of the area for more than 20,000 years. © UNESCO SUMMARY 2020 Conservation Outlook Finalised on 02 Dec 2020 GOOD WITH SOME CONCERNS There have been competing land and resource interests at all times along the boundaries of the Tasmanian Wilderness World Heritage site since inscription. Such competing interests have accompanied the various extensions in 1989 and more recently in 2010, 2012 and 2013. However, it is important to recall that the World Heritage Committee approved all these extensions brought forward by the State Party. These additional areas have consolidated the Outstanding Universal Value of the site. Even though the World Heritage site is in a privileged position due to its vast scale, active management, very limited access and harsh environmental conditions, it faces ongoing current and future threats. These include adequate and reliable resourcing, including for monitoring, commercial tourism interests and biosecurity risks and threats in the broadest sense. Climate change is an overarching concern and has plausibly been linked to already observable changes in fire frequency and intensity. These could pose significant threats to ancient (and other significant) life forms and landscapes that have been created, modified and managed by long term Aboriginal fire management and that form some of the key attributes of the World Heritage site. -

2531 WHA Man Folder Cover
Tasmanian Wilderness Tasmanian World Heritage Tasmanian Wilderness World Heritage Area MANAGEMENT PLAN 1999 Area Wilderness MANAGEMENT World Heritage PLAN 1999 Area “To identify, protect, conserve, present and, where appropriate, rehabilitate the world heritage and other natural and cultural values of the WHA, and to transmit that heritage to future generations in as good or better condition than at present.” WHA Management Plan, Overall Objective, 1999 MANAGEMENT PLAN PARKS PARKS and WILDLIFE and WILDLIFE SERVICE 1999 SERVICE ISBN 0 7246 2058 3 Tasmanian Wilderness World Heritage Area PARKS MANAGEMENT PLAN and WILDLIFE 1999 SERVICE TASMANIAN WILDERNESS WORLD HERITAGE AREA MANAGEMENT PLAN 1999 This management plan replaces the Tasmanian Abbreviations and General Terms Wilderness World Heritage Area Management The meanings of abbreviations and general terms Plan 1992, in accordance with Section 19(1) of the used throughout this plan are given below. National Parks and Wildlife Act 1970. A glossary of technical terms and phrases is The plan covers those parts of the Tasmanian provided on page 206. Wilderness World Heritage Area and 21 adjacent the Director areas (see table 2, page 15) reserved under the National Parks and Wildlife Act 1970 and has been The term ‘Director’ refers to the Director of prepared in accordance with the requirements of National Parks and Wildlife, a statutory position Part IV of that Act. held by the Director of the Parks and Wildlife Service. The draft of this plan (Tasmanian Wilderness World Heritage Area Management Plan 1997 the Minister Draft) was available for public comment from 14 The ‘Minister’ refers to the Minister administering November 1997 until 16 January 1998. -

Approved Conservation Advice for Niveoscincus Palfreymani (Pedra Branca Skink)
This Conservation Advice was approved by the Minister on 29 Apr 2014 Approved Conservation Advice for Niveoscincus palfreymani (Pedra Branca skink) (s266B of the Environment Protection and Biodiversity Conservation Act 1999) This Conservation Advice has been developed based on the best available information at the time this Conservation Advice was approved; this includes existing plans, records or management prescriptions for this species. Description Niveoscincus palfreymani (Pedra Branca skink), Family Scincidae, also known as the red- throated skink, is a moderately large and strongly built skink with well-developed limbs, a stout tail and smooth dorsal scales. The skinks vary in the intensity of colour and patterning. Adults can be grey, green or glossy black. Juveniles are light-tan, darkening on maturity. The head is variegated and spotted. A thin, light-coloured, dorso-lateral stripe exists in some specimens. There is a mid-lateral line apparent from behind the eye to the hind leg and below this the flanks are dark with scattered pale or bronze flecks. The ventral surface is grey with a tan to reddish tone. The adult males and females are similar in size with a mean snout–vent length of 88 mm and a mass of 17.7 g (Brothers and Pemberton, 1997). This species was previously known as Pseudomoia palfreymani (Cogger, 2000). Conservation Status The Pedra Branca skink is listed as vulnerable. This species is eligible for listing as vulnerable under the Environment Protection and Biodiversity Conservation Act 1999 (Cwlth) (EPBC Act) as, prior to the commencement of the EPBC Act, it was listed as vulnerable under Schedule 1 of the Endangered Species Protection Act 1992 (Cwlth). -

Foxreview Web
Invasive Animals Cooperative Research Centre Membership of Fox Review Team Dr Glen Saunders, Vertebrate Pest Research Unit, NSW Department of Primary Industries, Forest Rd., Orange, NSW 2800, Australia Mr Chris Lane, Invasive Animals Cooperative Research Centre, NSW Department of Primary Industries, Forest Rd., Orange, NSW 2800, Australia Professor Stephen Harris, School of Biological Sciences, University of Bristol, Woodlands Rd, Bristol BS8 1UG, United Kingdom Professor Chris Dickman, Institute of Wildlife Research, School of Biological Sciences, University of Sydney, Sydney, NSW 2006, Australia © Invasive Animals Cooperative Research Centre This work is copyright. The Copyright Act 1968 permits fair dealing for study, research, news reporting, criticism or review. Selected passages, tables or diagrams may be reproduced for such purposes provided acknowledgement of the source is included. Major extracts of the entire document may not be reproduced by any process. Published by the Invasive Animals Cooperative Research Centre ISBN 0-9775707-1-1 Copies may be requested from the Invasive Animals Cooperative Research Centre, University of Canberra, BELCONNEN ACT 2601 This publication should be cited as: Saunders, G., Lane, C., Harris, S., and Dickman, C. (2006). Foxes in Tasmania: a Report on an Incursion of an Invasive Species. 26 June 2006 Invasive Animals CRC Animals Invasive Foxes in Tasmania Contents Foreword . 1 Acknowledgements . 2 List of Abbreviations . 3 Terms of Reference . 4 Conclusions . 5 Key Recommendations . 6 The Report 1. Introduction and Background . 7 2. Legislation . 9 3. Chronology of Events . 10 4. Evidence . 18 5. Risk Analyses . 27 6. Fox Free Taskforce . 31 7. Fox Biology and Management . 39 8. Community and Communication .