Minutes of CHMP Written Procedure 14-17 August 2017 EMA/CHMP/467690/2017 Page 2/13 6
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytotoxic Chemotherapy: Still the Mainstay of Clinical Practice for All
G Model ONCH-2130; No. of Pages 14 ARTICLE IN PRESS Critical Reviews in Oncology/Hematology xxx (2016) xxx–xxx Contents lists available at ScienceDirect Critical Reviews in Oncology/Hematology journal homepage: www.elsevier.com/locate/critrevonc Cytotoxic chemotherapy: Still the mainstay of clinical practice for all subtypes metastatic breast cancer a,b b,∗ c c Chris Twelves , Maria Jove , Andrea Gombos , Ahmad Awada a Leeds Institute of Cancer and Pathology, St. James’s University Hospital, Leeds, UK b St James’s Institute of Oncology, St. James’s University Hospital, Bexley Wing, Level 4, Beckett Street, LS9 7TF Leeds, UK c Medical Oncology Clinic, Jules Bordet Institute, Université Libre de Bruxelles, Boulevard de Waterloo 121, B-1000 Brussels, Belgium Contents 1. Introduction . 00 2. Current therapeutic options for anthracycline- and taxane pretreated MBC . 00 2.1. Rechallenge with, or reformulation of, an anthracyclines or taxane . 00 2.2. Capecitabine . 00 3. Newer antimicrotubule agents . 00 3.1. Ixabepilone . 00 3.2. Eribulin . 00 4. Emerging new agents . 00 4.1. Vinflunine . 00 4.2. Etirinotecan pegol (NKTR-102). .00 4.2.1. Pharmacology. .00 4.2.2. Early clinical trials . 00 4.2.3. The BEACON trial . 00 5. Conclusions . 00 Conflict of interest . 00 Acknowledgments . 00 References . 00 Biography . 00 a r t i c l e i n f o a b s t r a c t Article history: Cytotoxic chemotherapy remains central to the treatment of all subtypes of metastatic breast cancer Received 13 October 2015 (MBC). We review evidence-based chemotherapy options for women with MBC after an anthracycline Received in revised form and a taxane including re-challenge with anthracycline or taxane, capecitabine, eribulin and ixabepilone 24 December 2015 as a single agent or combination with capecitabine (not approved in the EU); and the vinca alkaloid Accepted 20 January 2016 vinflunine as single agent or combined with either capecitabine/gemcitabine (also not approved EU or USA). -

Management of Brain and Leptomeningeal Metastases from Breast Cancer
International Journal of Molecular Sciences Review Management of Brain and Leptomeningeal Metastases from Breast Cancer Alessia Pellerino 1,* , Valeria Internò 2 , Francesca Mo 1, Federica Franchino 1, Riccardo Soffietti 1 and Roberta Rudà 1,3 1 Department of Neuro-Oncology, University and City of Health and Science Hospital, 10126 Turin, Italy; [email protected] (F.M.); [email protected] (F.F.); riccardo.soffi[email protected] (R.S.); [email protected] (R.R.) 2 Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro, 70121 Bari, Italy; [email protected] 3 Department of Neurology, Castelfranco Veneto and Treviso Hospital, 31100 Treviso, Italy * Correspondence: [email protected]; Tel.: +39-011-6334904 Received: 11 September 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: The management of breast cancer (BC) has rapidly evolved in the last 20 years. The improvement of systemic therapy allows a remarkable control of extracranial disease. However, brain (BM) and leptomeningeal metastases (LM) are frequent complications of advanced BC and represent a challenging issue for clinicians. Some prognostic scales designed for metastatic BC have been employed to select fit patients for adequate therapy and enrollment in clinical trials. Different systemic drugs, such as targeted therapies with either monoclonal antibodies or small tyrosine kinase molecules, or modified chemotherapeutic agents are under investigation. Major aims are to improve the penetration of active drugs through the blood–brain barrier (BBB) or brain–tumor barrier (BTB), and establish the best sequence and timing of radiotherapy and systemic therapy to avoid neurocognitive impairment. Moreover, pharmacologic prevention is a new concept driven by the efficacy of targeted agents on macrometastases from specific molecular subgroups. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

A Multicenter, Phase I, Dose-Escalation Study to Assess the Safety, Tolerability, and Pharmacokinetics of Etirinotecan Pegol in Patients with Refractory Solid Tumors
Published OnlineFirst November 7, 2012; DOI: 10.1158/1078-0432.CCR-12-1201 Clinical Cancer Cancer Therapy: Clinical Research A Multicenter, Phase I, Dose-Escalation Study to Assess the Safety, Tolerability, and Pharmacokinetics of Etirinotecan Pegol in Patients with Refractory Solid Tumors Gayle S. Jameson1, John T. Hamm3, Glen J. Weiss1, Carlos Alemany4, Stephen Anthony1, Michele Basche5, Ramesh K. Ramanathan1, Mitesh J. Borad2, Raoul Tibes2, Allen Cohn5, Ioana Hinshaw5, Robert Jotte5, Lee S. Rosen6, Ute Hoch7, Michael A. Eldon7, Robert Medve7, Katrina Schroeder1, Erica White1, and Daniel D. Von Hoff1 Abstract Purpose: This study was designed to establish the maximum tolerated dose (MTD) and to evaluate tolerability, pharmacokinetics, and antitumor activity of etirinotecan pegol. Experimental Design: Patients with refractory solid malignancies were enrolled and assigned to escalating-dose cohorts. Patients received 1 infusion of etirinotecan pegol weekly 3 times every 4 weeks (w  3q4w), or every 14 days (q14d), or every 21 days (q21d), with MTD as the primary end point using a standard 3 þ 3 design. Results: Seventy-six patients were entered onto 3 dosing schedules (58–245 mg/m2). The MTD was 115 mg/m2 for the w  3q4w schedule and 145 mg/m2 for both the q14d and q21d schedules. Most adverse events related to study drug were gastrointestinal disorders and were more frequent at higher doses of etirinotecan pegol. Late onset diarrhea was observed in some patients, the frequency of which generally correlated with dose density. Cholinergic diarrhea commonly seen with irinotecan treatment did not occur in patients treated with etirinotecan pegol. Etirinotecan pegol administration resulted in sustained and controlled systemic exposure to SN-38, which had a mean half-life of approximately 50 days. -

NKTR-102 Biomarker ASCO Poster
Etirinotecan pegol Target-Specific Pharmacodynamic (PD) Biomarkers Measured in Circulating Tumor Cells (CTCs) from Patients in the Phase 3 BEACON Study in Patients with Metastatic Breast Cancer (mBC) Ute Hoch,a Dennis G. Fry,a Yen Lin Chia,a Katie Caygill,a Alison L. Hannah,b Edith A. Perez,c Javier Cortez,d Ahmad Awada,e Joyce O'Shaughnessy,f Christopher Twelves,g Hope S. Rugo,h Seock-Ah Im,i Binghe Xu,j Kenna Anderes,k Darren W. Davis.k aNektar Therapeutics, San Francisco, CA; bConsultant, Sebastopol, CA; cMayo Clinic, Jacksonville, FL; dVall d'Hebron Institute of Oncoloy, Barcelona, Spain; eMedical Oncology Clinic, Jules Bordet Institute, Brussels, Belgium; fTexas Oncology–Baylor Charles A. Sammons Cancer Center, Dallas, TX; gUniversity of Leeds and St. James's University Hospital, Leeds, United Kingdom; hUniversity of California, San Francisco, CA; jSeoul National University Hospital, Seoul, South Korea; jCancer Hospital, Chinese Academy of Medical Sciences, Beijing, China; kApoCell, Inc., Houston, TX INTRODUCTION METHODS PHASE 3 BEACON • Etirinotecan pegol (EP, NKTR-102) is a unique long acting topoisomerase 1 inhibitor designed for prolonged tumor cell exposure. Primary and secondary antibodies were obtained from commercial sources.Control (0.1% DMSO) and drug-treated (SN38, 10 uM) tumor cell lines (HCT116, MCF7, A549, SKBr3) and PBMCs from healthy CTC Sampling Schedule donors were used for biomarker qualification. For each marker, 1-3 different antibodies were tested. Signals of biomarker staining were compared to secondary antibody staining only and iostype controls • In patients, etirinotecan pegol leads to greatly prolonged plasma SN38 exposure compared to irinotecan (elimination The CTC sampling schedule processed in the same experiment. -

Onzeald Preclinical Poster-2016SABCS
San Antonio Breast Cancer Symposium – December 6-10, 2016 P1-12-05 Etirinotecan Pegol: Survival Advantage Over Standard of Care Drugs in a Model of Brain Metastases of Breast Cancer Shah N1, Mohammad AS1, Adkins CE1, Dolan EL1, Grifth J1, Jagannathan R1, Ute Hoch2, and Lockman PR1 1Department of Pharmaceutical Sciences, School of Pharmacy, HSC, West Virginia University, 1 Medical Center Drive, Morgantown, WV, 26506-9050, USA; 2Nektar Therapeutics, 455 Mission Bay Blvd South, San Francisco, CA 94158, USA. Background Methods Results Novel Pharmacology of Etirinotecan Pegol • Efcacy Figure 2. Blood-Brain Barrier is Difcult to Penetrate 5 Table 1. Physical-Chemical Properties of Small Molecule Chemotherapies Figure 8. Etirinotecan Pegol Concentrates in Brain Tumor Tissue and Allows (NKTR-102) – Female athymic nude mice were inoculated with 1.75 x 10 MDA-MB-231-BrLuc for Metastatic Breast Cancer Compared to Etirinotecan Pegol Accumulation of SN38 Capillary in Healthy Brain Capillary in Brain with Tumor cells via intracardiac injection Molecular Efux Etirinotecan Pegol and SN38 Concentrations in Orthotopically (Brain) Implanted MDA-MB-231 • Compared to irinotecan, Irinotecan Basement Mitochondria Basement membrane membrane Mitochondria – Starting on day 21, gemcitabine (60 mg/kg) and eribulin (1.5 mg/kg) were dosed Weight (Da) Hydrophilic Transport Tumors After IV Administration of Etirinotecan Pegol etirinotecan pegol prolongs Ester-based Pinocytic Astrocyte More Astrocyte foot pinocytic IP every 4 days; etirinotecan pegol (50 mg/kg), irinotecan (50 mg/kg), paclitaxel 1 vessels foot Taxanes (paclitaxel, docetaxel) ~800 Hydrophobic Yes ) Single Dose Releasable vessels g elimination half-life of SN38 from Nucleus / 100 (6 mg/kg), vinorelbine (10 mg/kg), docetaxel (10 mg/kg), and vehicle (saline or g 0.1 1 Linker (n Eribulin 730 Hydrophobic Yes 2 days to 40 days in patients . -

Nonclinical Pharmacokinetics and Activity of Etirinotecan Pegol (NKTR-102), a Long-Acting Topoisomerase 1 Inhibitor, in Multiple Cancer Models
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Cancer Chemother Pharmacol (2014) 74:1125–1137 DOI 10.1007/s00280-014-2577-7 ORIGINAL ARTICLE Nonclinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models Ute Hoch · Carl-Michael Staschen · Randall K. Johnson · Michael A. Eldon Received: 15 April 2014 / Accepted: 27 August 2014 / Published online: 17 September 2014 © The Author(s) 2014. This article is published with open access at Springerlink.com Abstract of tumor growth. Etirinotecan pegol was eliminated very Purpose The aim of the study was to demonstrate the slowly from the tumor (t 17 days), achieving higher 1/2 = activity of etirinotecan pegol, a polymer conjugate of iri- and more sustained tumor exposure when compared with notecan, in multiple human tumor models and to establish conventional irinotecan. The increased tumor exposure fol- both the pharmacokinetic/pharmacodynamics (PK/PD) lowing etirinotecan pegol correlated with strong and pro- relationship and clinical relevance of the findings. longed suppression of tumor growth. Sustained plasma Experimental design Anti-tumor activity was evaluated in exposure to active SN38 was consistently observed across mouse models of human lung, colorectal, breast, ovarian, nonclinical species (including mouse, rat, and dog) and and gastric cancers. Etirinotecan pegol was administered translated to cancer patients. intravenously (once or every 3–7 days) to animals with Conclusions Etirinotecan pegol is the first long-acting established tumors. Activity was assessed by tumor growth topoisomerase 1 inhibitor that provides sustained exposure, delay (TGD) and regression. -

Prolonged Survival in Patients with Breast Cancer and a History of Brain
Breast Cancer Res Treat (2017) 165:329–341 DOI 10.1007/s10549-017-4304-7 CLINICAL TRIAL Prolonged survival in patients with breast cancer and a history of brain metastases: results of a preplanned subgroup analysis from the randomized phase III BEACON trial 1 2 3 4 Javier Corte´s • Hope S. Rugo • Ahmad Awada • Chris Twelves • 5 6 7 8 Edith A. Perez • Seock–Ah Im • Patricia Go´mez-Pardo • Lee S. Schwartzberg • 9 10 11 12 Veronique Die´ras • Denise A. Yardley • David A. Potter • Audrey Mailliez • 1 13 14 14 Alvaro Moreno-Aspitia • Jin-Seok Ahn • Carol Zhao • Ute Hoch • 14 15 16 Mary Tagliaferri • Alison L. Hannah • Joyce O’Shaughnessy Received: 12 May 2017 / Accepted: 16 May 2017 / Published online: 13 June 2017 Ó The Author(s) 2017. This article is an open access publication Abstract those with treated, stable brain metastases. The primary Purpose Conventional chemotherapy has limited activity endpoint, overall survival (OS), was assessed in a pre-de- in patients with breast cancer and brain metastases fined subgroup of BCBM patients; an exploratory post hoc (BCBM). Etirinotecan pegol (EP), a novel long-acting analysis adjusting for the diagnosis-specific graded prog- topoisomerase-1 inhibitor, was designed using advanced nostic assessment (GPA) index was also conducted. polymer technology to preferentially accumulate in tumor Results In the trial, 67 BCBM patients were randomized tissue including brain metastases, providing sustained (EP, n = 36; TPC, n = 31). Treatment subgroups were cytotoxic SN38 levels. balanced for baseline characteristics and GPA indices. EP Methods The phase 3 BEACON trial enrolled 852 women was associated with a significant reduction in the risk of with heavily pretreated locally recurrent or metastatic death (HR 0.51; P \ 0.01) versus TPC; median OS was breast cancer between 2011 and 2013. -
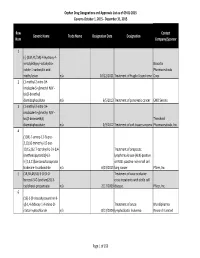
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd. -

13-400 Phrma Stateclinicaltrials MN 120913.Indd
Research in Your Backyard Developing Cures, Creating Jobs PHARMACEUTICAL CLINICAL TRIALS IN Dots show locations of clinical trials in the state. MINNESOTA Executive Summary Clinical Trials in Minnesota “ Advancements in medicine would not be • Biopharmaceutical research companies have possible without individuals willing to volunteer conducted more than 2,900 clinical trials of new their time to participate in clinical studies. medicines since 1999 in collaboration with the The Minnesota Clinical Research Association state’s local research institutions, including the Clinical Research Institute in Minneapolis and (MCRA) is a collaboration of Minnesota based Plymouth, Twin Cities Clinical Research/ research institutions and professionals who are Prism Clinical Research in Brooklyn Center, committed to objectively informing the general Prism Clinical Research in St. Paul, MAPS public to the benefits and risks involved in Applied Research Center in Edina, Minnesota clinical research far beyond international and Diet Research Center/Frestedt Inc. in St. Louis domestic regulations. MCRA members believe Park, Mercy Hospital in Coon Rapids, St. Mary’s that a fully informed participant is critical to Duluth Clinic in Duluth, St. Cloud Hospital in successful clinical research outcomes.” St. Cloud, St. Paul’s Heart Clinic in St. Paul, the — Jeff Cosgrove, President University of Minnesota School of Medicine in Prism Clinical Research Minneapolis and the Mayo Clinic in Rochester. • The trials have been aimed at a wide array of diseases and medical conditions, ranging from • MCRA provides important clinical research the most debilitating chronic diseases, including education at a time when it is a challenge for cancer, pain, heart disease, obesity and diabetes, to biopharmaceutical and device companies and bothersome allergies, sinusitis, chronic obstructive local research collaborators to recruit volunteers, pulmonary disease (COPD), migraine headaches, and patients often don’t know about trials of pneumonia and rare diseases affecting small new drugs. -

CHMP Agenda November 2017 for Publication
6 November 2017 EMA/CHMP/721012/2017 Inspections, Human Medicines Pharmacovigilance and Committees Division Committee for medicinal products for human use (CHMP) Draft agenda for the meeting on 06-09 November 2017 Chair: Tomas Salmonson – Vice-Chair: Harald Enzmann 06 November 2017, 13:00 – 19:30, room 2A 07 November 2017, 08:30 – 19:30, room 2A 08 November 2017, 08:30 – 19:30, room 2A 09 November 2017, 08:30 – 15:00, room 2A Health and safety information In accordance with the Agency’s health and safety policy, delegates are to be briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, this agenda is a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. -

Etirinotecan Pegol (NKTR-102) Passes Interim Efficacy Analysis for BEACON Pivotal Phase 3 Clinical Study in Patients with Metastatic Breast Cancer
January 14, 2014 Etirinotecan Pegol (NKTR-102) Passes Interim Efficacy Analysis for BEACON Pivotal Phase 3 Clinical Study in Patients with Metastatic Breast Cancer SAN FRANCISCO, Jan. 14, 2014 /PRNewswire/ -- Nektar Therapeutics (NASDAQ:NKTR) announced today that the Independent Data Monitoring Committee (DMC) created to provide safety oversight for the Company's pivotal clinical study for etirinotecan pegol (NKTR-102) has recommended continuation of the BEACON phase 3 trial, based upon the completion of a planned interim efficacy analysis in accordance with the DMC charter. The BEACON trial is evaluating NKTR-102 versus an agent of physician's choice for the treatment of locally recurrent or metastatic breast cancer, with a primary efficacy endpoint of overall survival. NKTR-102 is the first long-acting topoisomerase I-inhibitor designed to concentrate in tumor tissue, provide sustained tumor suppression throughout the entire chemotherapy cycle, and to reduce the peak exposures that are associated with toxicities of other cytotoxics. The independent DMC performed the pre-defined interim efficacy analysis, which consisted of a review of ongoing efficacy and safety data, including 50% of patient events necessary to evaluate the primary endpoint of overall survival. In August 2013, the BEACON study completed enrollment of 852 patients with advanced breast cancer whose disease has progressed following treatment with anthracycline, taxane and capecitabine therapies (ATC). "While the results of BEACON remain blinded to Nektar, we are very pleased that the NKTR-102 trial has successfully passed this important interim efficacy analysis," said Howard Robin, President and CEO of Nektar Therapeutics. "We expect final results from the study at the end of 2014 or early 2015, and if positive, we plan to submit filings in the U.S.