Collection and Analysis of Volatiles of Various Cultivated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scientific Name Species Common Name Abies Lasiocarpa FIR Subalpine Acacia Macracantha ACACIA Long-Spine
Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek Alnus iridis ssp. sinuata ALDER Sitka Alnus maritima ALDER Seaside Alvaradoa amorphoides ALVARADOA Mexican Amelanchier laevis SERVICEBERRY Allegheny Amyris balsamifera TORCHWOOD Balsam Annona squamosa SUGAR-APPLE NA Araucaria cunninghamii ARAUCARIA Cunningham Arctostaphylos glauca MANZANITA Bigberry Asimina obovata PAWPAW Bigflower Bourreria radula STRONGBACK Rough Brasiliopuntia brasiliensis PRICKLY-PEAR Brazilian Bursera simaruba GUMBO-LIMBO NA Caesalpinia pulcherrima FLOWERFENCE NA Capparis flexuosa CAPERTREE Limber CRUCIFIXION- Castela emoryi THORN NA Casuarina equisetifolia CASUARINA Horsetail Ceanothus arboreus CEANOTHUS Feltleaf Ceanothus spinosus CEANOTHUS Greenbark Celtis lindheimeri HACKBERRY Lindheimer Celtis occidentalis HACKBERRY Common Cephalanthus occidentalis BUTTONBUSH Common Cercis canadensis REDBUD Eastern Cercocarpus traskiae CERCOCARPUS Catalina Chrysophyllum oliviforme SATINLEAF NA Citharexylum berlandieri FIDDLEWOOD Berlandier Citrus aurantifolia LIME NA Citrus sinensis ORANGE Orange Coccoloba uvifera SEAGRAPE NA Colubrina arborescens COLUBRINA Coffee Colubrina cubensis COLUBRINA Cuba Condalia globosa -

Biology and Integrated Pest Management of the Sunflower Stem
E-821 (Revised) Biology and Integrated Pest Management of the SunflowerSunflower StemStem WeevilsWeevils inin thethe GreatGreat PlainsPlains Janet J. Knodel, Crop Protection Specialist Laurence D. Charlet, USDA, ARS Research Entomologist he sunflower stem weevil, Cylindrocopturus adspersus T(LeConte), is an insect pest that has caused economic damage to sunflower in the northern and southern Great Plains of the USA and into Canada. It belongs in the order Coleoptera (beetles) and family Curculionidae (weevils), and has also been called the spotted sunflower stem weevil. It is native to North America and has adapted to wild and cultivated Figure 1. Damage caused by sunflower stem weevil – sunflower lodging and stalk breakage. sunflowers feeding on the stem and leaves. The sunflower stem weevil was first reported as a pest in 1921 from severely wilted plants in fields grown for silage in Colorado. In North Dakota, the first sunflower stem weevil infestation ■ Distribution was recorded in 1973, causing 80% The sunflower stem weevil has been reported from most states yield loss due to lodging (Figure 1). west of the Mississippi River and into Canada. Economically Populations of sunflower stem weevil damaging populations have been recorded in Colorado, Kansas, have fluctuated over the years with high Nebraska, North Dakota, Minnesota, South Dakota, and Texas. numbers in some areas from the 1980s The black sunflower stem weevil can be found in most sunflower production areas with the greatest concentrations in to early 1990s in North Dakota. southern North Dakota and South Dakota. Another stem feeding weevil called the black sunflower stem weevil, Apion occidentale Fall, also occurs throughout the Great Plains, and attacks sunflower as a host. -

Dry White Pine (Hemlock) Oak Forest
Dry white pine (hemlock) oak forest This community type occurs on fairly dry sites, often with 25% or more of the forest floor covered by rocks, boulders and/or exposed bedrock. The canopy may be somewhat open and tree growth somewhat suppressed. The tree stratum is dominated by a mixture of Pinus strobus (eastern white pine), or occasionally Tsuga canadensis (eastern hemlock), and a mixture of dry-site hardwoods, predominantly oaks. On most sites, the conifer and the hardwood component both range between 25% and 75% of the canopy. The oak species most often associated with this type are Quercus montana (chestnut oak), and Q. alba (white oak), although Q. velutina (black oak), Q. coccinea (scarlet oak), or Q. rubra (northern red oak) may also occur. Other associated trees include Nyssa sylvatica (black-gum), Betula lenta (sweet birch), Fraxinus americana (white ash), Prunus serotina (wild black cherry), and Castanea dentata (American chestnut) sprouts. There is often a heath-dominated shrub layer with Kalmia latifolia (mountain laurel) being especially important; Gaylussacia baccata (black huckleberry), Vaccinium spp. (blueberries), and Kalmia angustifolia (sheep laurel) are also common. Other shrubs, like Cornus florida (flowering dogwood), Hamamelis virginiana (witch-hazel), Viburnum acerifolium (maple-leaved viburnum) may also occur on less acidic sites. There is typically a sparse herbaceous layer with a northern affinity; Aralia nudicaulis (wild sarsaparilla), Pteridium aquilinum (bracken fern), Maianthemum canadense (Canada mayflower), Gaultheria procumbens (teaberry), Trientalis borealis (star-flower), and Medeola virginiana (Indian cumber-root) are typical. The successional status of this type seems variable, in some cases, especially on harsher sites, it appears relatively stable, in other cases it appears to be transitional. -

197 Section 9 Sunflower (Helianthus
SECTION 9 SUNFLOWER (HELIANTHUS ANNUUS L.) 1. Taxonomy of the Genus Helianthus, Natural Habitat and Origins of the Cultivated Sunflower A. Taxonomy of the genus Helianthus The sunflower belongs to the genus Helianthus in the Composite family (Asterales order), which includes species with very diverse morphologies (herbs, shrubs, lianas, etc.). The genus Helianthus belongs to the Heliantheae tribe. This includes approximately 50 species originating in North and Central America. The basis for the botanical classification of the genus Helianthus was proposed by Heiser et al. (1969) and refined subsequently using new phenological, cladistic and biosystematic methods, (Robinson, 1979; Anashchenko, 1974, 1979; Schilling and Heiser, 1981) or molecular markers (Sossey-Alaoui et al., 1998). This approach splits Helianthus into four sections: Helianthus, Agrestes, Ciliares and Atrorubens. This classification is set out in Table 1.18. Section Helianthus This section comprises 12 species, including H. annuus, the cultivated sunflower. These species, which are diploid (2n = 34), are interfertile and annual in almost all cases. For the majority, the natural distribution is central and western North America. They are generally well adapted to dry or even arid areas and sandy soils. The widespread H. annuus L. species includes (Heiser et al., 1969) plants cultivated for seed or fodder referred to as H. annuus var. macrocarpus (D.C), or cultivated for ornament (H. annuus subsp. annuus), and uncultivated wild and weedy plants (H. annuus subsp. lenticularis, H. annuus subsp. Texanus, etc.). Leaves of these species are usually alternate, ovoid and with a long petiole. Flower heads, or capitula, consist of tubular and ligulate florets, which may be deep purple, red or yellow. -

Specializing in Rare and Unique Trees 2015 Catalogue
Whistling Gardens 698 Concession 3, Wilsonville, ON N0E 1Z0 Phone- 519-443-5773 Fax- 519-443-4141 Specializing in Rare and Unique Trees 2015 Catalogue Pot sizes: The number represents the size of the pot ie. #1= 1 gallon, #10 = 10 gallon #1 potted conifers are usually 3-5years old. #10 potted conifers dwarf conifers are between 10 and 15 years old #1 trees= usually seedlings #10 trees= can be several years old anywhere from 5 to 10' tall depending on species and variety. Please ask us on sizes and varieties you are not sure about. Many plants are limited to 1specimen. To reserve your plant(s) a 25% is required. Plants should be picked up by June 15th. Most plants arrive at the gardens by May 10th. Guarantee: We cannot control the weather (good or bad), rodents (big or small), pests (teenie, tiny), poor siting, soil types, lawnmovers, snowplows etc. Plants we carry are expected to grow within the parameters of normal weather conditons. All woody plant purchases are guaranteed from time of purchase to December 1st of current year Any plant not performing or dying in current season will be happily replaced or credited towards a new plant. Please email us if possible with any info needed about our plants. We do not have a phone in the garden centre and I'm rarely in the office. It is very helpful to copy and paste the botanical name of the plant into your Google browser, in most cases, a d Order Item Size Price Description of Pot European White Fir Zone 5 Abies alba Barabit's Spreader 25-30cm #3 $100.00 Abies alba Barabit's Spreader 40cm -

Deer-Resistant Landscape Plants This List of Deer-Resistant Landscape Plants Was Compiled from a Variety of Sources
Deer-Resistant Landscape Plants This list of deer-resistant landscape plants was compiled from a variety of sources. The definition of a deer-resistant plant is one that may be occasionally browsed, but not devoured. If deer are hungry enough or there’s a limited amount of food available, they will eat almost anything. Perennials, Herbs & Bulbs Achillea Yarrow Lavandula augustifolia Lavender Adiatum pedatum Maiden Hair Fern Liatris Gayfeather Agastache cana Agastache Lychnis chalcedonica Maltese Cross Ajuga reptans Bugleweed Matteuccia Ostrich Fern Alchemilla Lady’s Mantle Mentha Mint Allium spp. Flowering Onion Miscanthus Silver Grass Arabis Rock Cress Myosotis sylvatica Forget-Me-Not Armeria maritime Sea Thrift Narcissus Daffodils & Narcissus Artemisia Wormwood Nepeta Catmint Asarum europaeum Wild Ginger Oenothera Evening Primrose Asclepias tuberosa Butterfly Weed Paeonia Peony Bergenia Bergenia Papaver orientale Oriental Poppy Calamagrostis Reed Grass Pennisetum orientale Oriental Fountain Grass Cerastium Snow-In-Summer Perovskia Russian Sage Cimcifuga Bugbane Physostegia Obedient Plant Convallaria Lily-of-the-Valley Platycodon Balloon Flower Corydalis lutea Gold Bleeding Heart Polygonatum Solomon’s Seal Dicentra Bleeding Heart Pulmonaria Lungwort Digitalis Foxglove Rudbeckia Black Eyed Susan Echinops Globe Thistle Saponaria Soapwort Festuca ovinia Blue Fescue Salvia Sage Galanthus nivalis Snowdrop Sedum Stonecrop Helleborus Hellebores Solidago Goldenrod Iris sibirica & ensata Japanese & Siberian Iris Stachys byzantina Lamb’s Ear Lamium -

Control Biológico De Insectos: Clara Inés Nicholls Estrada Un Enfoque Agroecológico
Control biológico de insectos: Clara Inés Nicholls Estrada un enfoque agroecológico Control biológico de insectos: un enfoque agroecológico Clara Inés Nicholls Estrada Ciencia y Tecnología Editorial Universidad de Antioquia Ciencia y Tecnología © Clara Inés Nicholls Estrada © Editorial Universidad de Antioquia ISBN: 978-958-714-186-3 Primera edición: septiembre de 2008 Diseño de cubierta: Verónica Moreno Cardona Corrección de texto e indización: Miriam Velásquez Velásquez Elaboración de material gráfico: Ana Cecilia Galvis Martínez y Alejandro Henao Salazar Diagramación: Luz Elena Ochoa Vélez Coordinación editorial: Larissa Molano Osorio Impresión y terminación: Imprenta Universidad de Antioquia Impreso y hecho en Colombia / Printed and made in Colombia Prohibida la reproducción total o parcial, por cualquier medio o con cualquier propósito, sin autorización escrita de la Editorial Universidad de Antioquia. Editorial Universidad de Antioquia Teléfono: (574) 219 50 10. Telefax: (574) 219 50 12 E-mail: [email protected] Sitio web: http://www.editorialudea.com Apartado 1226. Medellín. Colombia Imprenta Universidad de Antioquia Teléfono: (574) 219 53 30. Telefax: (574) 219 53 31 El contenido de la obra corresponde al derecho de expresión del autor y no compromete el pensamiento institucional de la Universidad de Antioquia ni desata su responsabilidad frente a terceros. El autor asume la responsabilidad por los derechos de autor y conexos contenidos en la obra, así como por la eventual información sensible publicada en ella. Nicholls Estrada, Clara Inés Control biológico de insectos : un enfoque agroecológico / Clara Inés Nicholls Estrada. -- Medellín : Editorial Universidad de Antioquia, 2008. 282 p. ; 24 cm. -- (Colección ciencia y tecnología) Incluye glosario. Incluye bibliografía e índices. -
Trees & Conifers
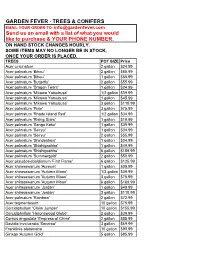
GARDEN FEVER - TREES & CONIFERS EMAIL YOUR ORDER TO: [email protected] Send us an email with a list of what you would like to purchase & YOUR PHONE NUMBER. ON HAND STOCK CHANGES HOURLY. SOME ITEMS MAY NO LONGER BE IN STOCK, ONCE YOUR ORDER IS PLACED. TREES POT SIZE Price Acer ciricinatum 2 gallon $24.99 Acer palmatum 'Bihou' 2 gallon $85.99 Acer palmatum 'Bihou' 1 gallon $55.99 Acer palmatum 'Butterfly' 2 gallon $55.99 Acer palmatum 'Dragon Tears' 1 gallon $24.99 Acer palmatum 'Mikawa Yatsubusa' 1/2 gallon $39.99 Acer palmatum 'Mikawa Yatsubusa' 1 gallon $45.99 Acer palmatum 'Mikawa Yatsubusa' 3 gallon $110.99 Acer palmatum 'Pixie' 3 gallon $75.99 Acer palmatum 'Rhode Island Red' 1/2 gallon $34.99 Acer palmatum 'Rising Stars' 1 gallon $19.99 Acer palmatum 'Sango Kaku' 1 gallon $39.99 Acer palmatum 'Seiryu' 1 gallon $34.99 Acer palmatum 'Seiryu' 2 gallon $55.99 Acer palmatum 'Shindeshojo' 1 gallon $34.99 Acer palmatum 'Shishigashira' 1 gallon $49.99 Acer palmatum 'Shishigashira' 6 gallon $159.99 Acer palmatum 'Summergold' 2 gallon $59.99 Acer pseudosieboldianum 'First Flame' 6 gallon $125.99 Acer shirasawanum 'Aureum' 1 gallon $39.99 Acer shirasawanum 'Autumn Moon' 1/2 gallon $39.99 Acer shirasawanum 'Autumn Moon' 3 gallon $75.99 Acer shirasawanum 'Autumn Moon' 6 gallon $169.99 Acer shirasawanum 'Jordan' 1 gallon $49.99 Acer shirasawanum 'Jordan' 3 gallon $110.99 Acer palmatum 'Rainbow' 2 gallon $72.99 Acer tegmentosum 10 gallon $75.99 Cercidiphyllum 'Claim Jumper' 10 gallon $155.99 Cercidiphyllum 'Heronswood Globe' 2 gallon $39.99 Cornus -

Nursery Catalog
Tel: 503.628.8685 Fax: 503.628.1426 www.eshraghinursery.com 1 Eshraghi’s TOP 10 picks Our locations 1 Main Office, Shipping & Growing 2 Retail Store & Growing 26985 SW Farmington Road Farmington Gardens Hillsboro, OR 97123 21815 SW Farmington Road Beaverton, OR 97007 1 2 3 7 6 3 River Ranch Facility 4 Liberty Farm 4 5 10 N SUNSET HWY TO PORTLAND 8 9 TU HILLSBORO ALA TIN 26 VALL SW 185TH AVE. EY HWY. #4 8 BEAVERTON TONGUE LN. GRABEL RD . D R . E D G R ID E ALOHA R G B D I R R #3 SW 209TH E B T D FARMINGTON ROAD D N A I SIMPSON O O M O R R 10 217 ROSEDALE W R E S W V S I R N W O 219 T K C A J #2 #1 SW UNGER RD. SW 185TH AVE. 1 Acer circinatum ‘Pacific Fire’ (Vine Maple), page 6 D A SW MURRAY BLVD. N RO 2 palmatum (Japanese Maple), NGTO Acer 'Geisha Gone Wild' page 8 FARMI 3 Acer palmatum 'Mikawa yatsubusa' (Japanese Maple), page 10 #1 4 Acer palmatum dissectum 'Orangeola' (Japanese Maple), page 14 5 Hydrangea macrophylla 'McKay', Cherry Explosion PP28757 (Hydrangea), page 32 6 Picea glauca 'Eshraghi1', Poco Verde (White Spruce), page 61 ROAD HILL CLARK 7 Picea pungens 'Hockersmith', Linda (Colorado Spruce), page 64 RY ROAD 8 Pinus nigra 'Green Tower' (Austrian Pine), page 65 SCHOLLS FER 9 Thuja occidentalis 'Janed Gold', Highlights™ PP21967 (Arborvitae), page 70 10 Thuja occidentalis 'Anniek', Sienna Sunset™ (Arborvitae), page 69 Table of contents Tags Make a Difference . -

Hidden Lake Gardens Michigan State University 2019 Plant Sale Plant
Hidden Lake Gardens Michigan State University 2019 Plant Sale Plant List **Please Note: All plants listed are subject to availability due to weather conditions, crop failure, or supplier delivery** Vegetables and Herbs Many assorted varieties Annuals Assorted hanging baskets – Proven Winners® Selections Senecio ‘Angel Wings’ Supertunia® ‘Bordeaux’™ Supertunia® ‘Piccaso in Purple’® Supertunia Royale® Plum Wine Gerbera ‘Garvinea Sweet Sunset’ Salvia ‘Black and Bloom’ Coleus ‘Golden Gate’ Stained Glassworks™ Coleus ‘Royalty’ Stained Glassworks™ Coleus ‘Eruption’ Stained Glassworks™ Coleus ‘Luminesce’ Stained Glassworks™ Coleus ‘Great Falls Niagara’ Strobilanthes dyeriana Persian Shield Bidens ferulifolia ‘Bee Alive’ Bidens ferulifolia ‘Bee Happy’ Bidens ferulifolia ‘Sun Drop Compact’ Zinnia Zahara™ Lantana Lucky™ Angelonia angustifolia Archangel™ Portulaca ColorBlast™ ‘Watermelon Punch’ Perennials Achillea ‘Pomegranate’ Aconitum arendsii Agastache ‘Kudos Coral’ Agastache ‘Kudos Mandarin’ Aquilegia ‘Clementine Dark Purple’ Aquilegia ‘Clementine Salmon Rose’ Armeria maritima ‘Splendens’ Asclepias tuberosa Astilbe ‘Montgomery’ Astilbe ‘Visions’ (purple) Baptisia ‘Lactea’ Baptisia ‘Pink Lemonade’ Bergenia ‘Bressingham Ruby’ Bergenia ‘Sakura’ Brunnera macrophylla ‘Alexander’s Great’ Brunnera macrophylla ‘Jack Frost’ Brunnera macrophylla ‘Sea Heart’ Centaurea montana Ceratostigma plumbaginoides Chelone ‘Hot Lips’ Coreopsis ‘American Dream’ Coreopsis ‘Big Bang Galaxy’ Crocosmia ‘Lucifer’ Dianthus ‘Firewitch’ Dianthus x ‘Kahori’ Dicentra ‘Fire Island’ -

Journal of Hymenoptera Research
c 3 Journal of Hymenoptera Research . .IV 6«** Volume 15, Number 2 October 2006 ISSN #1070-9428 CONTENTS BELOKOBYLSKIJ, S. A. and K. MAETO. A new species of the genus Parachremylus Granger (Hymenoptera: Braconidae), a parasitoid of Conopomorpha lychee pests (Lepidoptera: Gracillariidae) in Thailand 181 GIBSON, G. A. P., M. W. GATES, and G. D. BUNTIN. Parasitoids (Hymenoptera: Chalcidoidea) of the cabbage seedpod weevil (Coleoptera: Curculionidae) in Georgia, USA 187 V. Forest GILES, and J. S. ASCHER. A survey of the bees of the Black Rock Preserve, New York (Hymenoptera: Apoidea) 208 GUMOVSKY, A. V. The biology and morphology of Entedon sylvestris (Hymenoptera: Eulophidae), a larval endoparasitoid of Ceutorhynchus sisymbrii (Coleoptera: Curculionidae) 232 of KULA, R. R., G. ZOLNEROWICH, and C. J. FERGUSON. Phylogenetic analysis Chaenusa sensu lato (Hymenoptera: Braconidae) using mitochondrial NADH 1 dehydrogenase gene sequences 251 QUINTERO A., D. and R. A. CAMBRA T The genus Allotilla Schuster (Hymenoptera: Mutilli- dae): phylogenetic analysis of its relationships, first description of the female and new distribution records 270 RIZZO, M. C. and B. MASSA. Parasitism and sex ratio of the bedeguar gall wasp Diplolqjis 277 rosae (L.) (Hymenoptera: Cynipidae) in Sicily (Italy) VILHELMSEN, L. and L. KROGMANN. Skeletal anatomy of the mesosoma of Palaeomymar anomalum (Blood & Kryger, 1922) (Hymenoptera: Mymarommatidae) 290 WHARTON, R. A. The species of Stenmulopius Fischer (Hymenoptera: Braconidae, Opiinae) and the braconid sternaulus 316 (Continued on back cover) INTERNATIONAL SOCIETY OF HYMENOPTERISTS Organized 1982; Incorporated 1991 OFFICERS FOR 2006 Michael E. Schauff, President James Woolley, President-Elect Michael W. Gates, Secretary Justin O. Schmidt, Treasurer Gavin R. -

Native Trees of Massachusetts
Native Trees of Massachusetts Common Name Latin Name Eastern White Pine Pinus strobus Common Name Latin Name Mountain Pine Pinus mugo Pin Oak Quercus palustris Pitch Pine Pinus rigida White Oak Quercus alba Red Pine Pinus resinosa Swamp White Oak Quercus bicolor Scots Pine Pinus sylvestris Chestnut Oak Quercus montana Jack Pine Pinus banksiana Eastern Cottonwood Populus deltoides Eastern Hemlock Tsuga canadensis https://plants.usda.gov/ Tamarack Larix laricina core/profile?symbol=P Black Spruce Picea mariana ODE3 White Spruce Picea glauca black willow Salix nigra Red Spruce Picea rubens Red Mulberry Morus rubra Norway Spruce Picea abies American Plum Prunus americana Northern White cedar Thuja occidentalis Canada Plum Prunus nigra Eastern Juniper Juniperus virginiana Black Cherry Prunus serotina Balsam Fir Abies balsamea Canadian Amelanchier canadensis American Sycamore Platanus occidentalis Serviceberry or Witchhazel Hamamelis virginiana Shadbush Honey Locust Gleditsia triacanthos American Mountain Sorbus americana Eastern Redbud Cercis canadensis Ash Yellow-Wood Cladrastis kentukea American Elm Ulmus americana Gray Birch Betula populifolia Slippery Elm Ulmus rubra Grey Alder Alnus incana Basswood Tilia americana Sweet Birch Betula lenta Smooth Sumac Rhus glabra Yellow Birch Betula alleghaniensis Red Maple Acer rubrum Heartleaf Paper Birch Betula cordifolia Horse-Chestnut Aesculus hippocastanum River Birch Betula nigra Staghorn Sumac Rhus typhina Smooth Alder Alnus serrulata Silver Maple Acer saccharinum American Ostrya virginiana Sugar Maple Acer saccharum Hophornbeam Boxelder Acer negundo American Hornbeam Carpinus caroliniana Black Tupelo Nyssa sylvatica Green Alder Alnus viridis Flowering Dogwood Cornus florida Beaked Hazelnut Corylus cornuta Northern Catalpa Catalpa speciosa American Beech Fagus grandifolia Black Ash Fraxinus nigra Black Oak Quercus velutina Devil's Walkingstick Aralia spinosa Downy Hawthorn Crataegus mollis.