10.29199-ARMS-101013.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NGRI Study Report in Rasipalayam Coimbatore District
Final Report Identification of source(s) of pollution (high TDS) in groundwater in north of Rasipalayam village, Sulur Taluk, Coimbatore district, Tamil Nadu A Project Sponsored by TNPCB CSIR-National Geophysical Research Institute Uppal Road, Hyderabad – 500007 April 2018 Technical Report No.: NGRI-2018-GW-956 Identification of source(s) of pollution (high TDS) in groundwater in north of Rasipalayam village, Sulur Taluk, Coimbatore district, Tamil Nadu Project Team Dr. Devender Kumar Dr. K. Rama Mohan Mr. B. Kiran Kumar & Dr. D. V. Reddy (Project Adviser) CSIR-National Geophysical Research Institute Uppal Road, Hyderabad – 500007 April 2018 Acknowledgements We would like to thank the Tamil Nadu Pollution Control Board (TNPCB) for funding this project and extending all possible help in completing the same. We also thank Director, CSIR-National Geophysical Research Institute, Hyderabad for his constant encouragement and support during the execution of the project. We are highly grateful to Dr. R. Rajamanickam, TNCPB, Chennai for his timely help. We place on record our sincere thanks to Mr. P. Manimaran, DEE, and Mr. K. Manivannan AEE, TNCPB, Coimbatore for their support to carry out the field work. We gratefully acknowledge the help provided by the local people of the area, specially Mr. A. K. Jaganathan, Mr. K. Madhan Kumar, Mr. G. Kanda Samy, Mr. V. Vishwanathan and Mr. Gunasekar Chandran. We thank Ms K. Gayathri of CSIR-NGRI for assisting in analysis of samples by Ion-Chromatography. Dr. N. C. Mondal deserves profound thanks for critically reviewing this report. Contents 1. Background 1 2. Objectives 1 3. Approaches 4. Reconnaissance and Preliminary Assessment 2 5. -

Coimbatore Commissionerate Jurisdiction
Coimbatore Commissionerate Jurisdiction The jurisdiction of Coimbatore Commissionerate will cover the areas covering the entire Districts of Coimbatore, Nilgiris and the District of Tirupur excluding Dharapuram, Kangeyam taluks and Uthukkuli Firka and Kunnathur Firka of Avinashi Taluk * in the State of Tamil Nadu. *(Uthukkuli Firka and Kunnathur Firka are now known as Uthukkuli Taluk). Location | 617, A.T.D. STR.EE[, RACE COURSE, COIMBATORE: 641018 Divisions under the jurisdiction of Coimbatore Commissionerate Sl.No. Divisions L. Coimbatore I Division 2. Coimbatore II Division 3. Coimbatore III Division 4. Coimbatore IV Division 5. Pollachi Division 6. Tirupur Division 7. Coonoor Division Page 47 of 83 1. Coimbatore I Division of Coimbatore Commissionerate: Location L44L, ELGI Building, Trichy Road, COIMBATORT- 641018 AreascoveringWardNos.l to4,LO to 15, 18to24and76 to79of Coimbatore City Municipal Corporation limit and Jurisdiction Perianaickanpalayam Firka, Chinna Thadagam, 24-Yeerapandi, Pannimadai, Somayampalayam, Goundenpalayam and Nanjundapuram villages of Thudiyalur Firka of Coimbatore North Taluk and Vellamadai of Sarkar Samakulam Firka of Coimbatore North Taluk of Coimbatore District . Name of the Location Jurisdiction Range Areas covering Ward Nos. 10 to 15, 20 to 24, 76 to 79 of Coimbatore Municipal CBE Corporation; revenue villages of I-A Goundenpalayam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore 5th Floor, AP Arcade, District. Singapore PIaza,333 Areas covering Ward Nos. 1 to 4 , 18 Cross Cut Road, Coimbatore Municipal Coimbatore -641012. and 19 of Corporation; revenue villages of 24- CBE Veerapandi, Somayampalayam, I-B Pannimadai, Nanjundapuram, Chinna Thadagam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore District. Areas covering revenue villages of Narasimhanaickenpalayam, CBE Kurudampalayam of r-c Periyanaickenpalayam Firka of Coimbatore North Taluk of Coimbatore District. -
![[Revised – 1960] MILITARY ENGINEER SERVIC](https://docslib.b-cdn.net/cover/7185/revised-1960-military-engineer-servic-787185.webp)
[Revised – 1960] MILITARY ENGINEER SERVIC
CA NO : GE (AF)/SUL/TOKEN NO 80 OF 2018-19 Serial Page No [In lieu of IAFW – 2162] [Revised – 1960] MILITARY ENGINEER SERVICES NOTICE INVITING TENDER 1. An e-Tender is invited for the work as mentioned in Appendix "A" to this Notice Inviting Tender [NIT]. 2. The work is estimated to cost as indicated in aforesaid Appendix "A". The estimate, however, is not a guarantee and is merely given as rough guide. If the work cost more or less, the tenderer / bidder will have no claim on this account. The tender shall be based on as mentioned in aforesaid Appendix "A". 3. The work is to be completed within the period as indicated in aforesaid Appendix "A" in accordance with the phasing if any, indicated in the tender from the date of handing over of site, which will be about two weeks after the date of acceptance of the tender. 4. Normally contractors whose names are on the MES approved list for the area in which the work lies and within whose financial category the estimated amount would fall may tender / bid. But in case of term contracts, contractors in categories "SS" to "E" may tender / bid. In case, where the tender amount is in excess of the financial limit of the contractor [i.e. his class of enlistment] and the Accepting Officer decides to accept the tender / bid, in which event the tenderer / bidder would be required to lodge "Additional Security Deposit" as notified by the Accepting Officer in terms of conditions of Contract. Contractors whose names are on the MES approved list of any MES formation and who have deposited Standing Security Deposit and have executed Standing Security Bond may also tender without depositing Earnest Money along with the tender / bid and if the tender / bid submitted by such a tenderer / bidder is accepted, the tenderer / bidder will be required to lodge with the Controller of Defence Accounts concerned the amount of "Individual Security Deposit" within thirty days of the receipt by him of notification of acceptance of his tender / bid, failing which the sum will be recovered from the first RAR payment or from the first final bill. -

Plan on Artificial Recharge to Groundwater and Water Conservation in Sulur Firka, Sulur Taluk, Coimbatore District, Tamil Nadu
Plan on Artificial Recharge to Groundwater and Water Conservation in Sulur Firka, Sulur Taluk, Coimbatore District, Tamil Nadu By Central Ground Water Board South Eastern Coastal Region Rajaji Bhawan, Besant Nagar Chennai Content S.No. TOPIC At a Glance 1 Introduction 2 Objectives 3. Study area details 3.1 Location 3.2 Geomorphological Setup 3.3 Landuse and Soil 3.4 Drainage 3.5 Rainfall 3.6 Hydrogeology 3.7 Dynamic Ground water Resources 4 Spatial data integration/ conservation 5 Planning for recharge 5.1 Justification of the artificial recharge 5.2 Availability of surplus surface water for artificial recharge or conservation 5.3 Proposed interventions including tentative location of artificial recharge structures and water conservation 5.3.1 Artificial recharge 5.3.1.1 Check Dam /Nala Bund 5.3.1.3. Revival, repair of water bodies 5.3.2. Water Conservation Measure 5.3.2.1 Farm Pond 5.3.2.2 Micro irrigation system 6. Tentative Cost Estimation 7. Implementation modalities a) Time schedule b) Operation and Maintenance AT GLANCE Name of Firka Sulur Taluk Sulur District Coimbatore State Tamil Nadu Total area (Sq.Kms) 117.61305 Total Area suitable for recharge 87.03 Co-ordinates: 10°56’45“to 11°04’40” & 77°02’09”to 77°10’33”. (Latitude. & Longitude) Rainfall 569 mm Monsoon 416 mm Non- Mon soon 153 mm Geology Crystalline and metamorphic gneiss complex of Archaean age WATER LEVEL Pre - Monsoon 2.2 to 18.56 m bgl. Post - Monsoon 1.4 to 11.20 m bgl. GROUND WATER RESOURCES ESTIMATION Replenish able ground water resources 9.93217 MCM Net ground -

DISTRICT SURVEY REPORT for GRAVEL and BRICK EARTH COIMBATORE DISTRICT Page Chapter Content No
DISTRICT SURVEY REPORT FOR GRAVEL AND BRICK EARTH COIMBATORE DISTRICT (Prepared as per Gazette Notification S.O.3611 (E) Dated 25.07.2018 of Ministry of Environment, Forest and Climate Change MoEF& CC) MAY 2019 DISTRICT SURVEY REPORT FOR GRAVEL AND BRICK EARTH COIMBATORE DISTRICT Page Chapter Content No. 1. Introduction 1 2. Overview of Mining Activity in the District 4 3. General Profile of the District 4 4. Geology of the District 8 5. Drainage of Irrigation pattern 11 6. Land Utilisation Pattern in the District: Forest, Agricultural, 13 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 15 8. Rainfall of the District and Climatic conditions 16 9. a) Occurrence of Gravel and Brick Earth in the Coimbatore District 16 b) Details of the mining/quarry leases in the District as per the following 17 format Details of Seigniorage feeReceived in last three years (2016-17 to 2018- 10. 18 19) 11. Details of Production in last three years (2016-17 to 2018-19) 18 12. Mineral map of the District 19 List of Letter of Intent (LOI) Holders in the District along with its 13. 20 validity as per the following format 14. Total mineral reserves available in the District 20 15. Quality/ Grade of Mineral available in the District 20 16. Use of Mineral 21 17. Demand and supply of the Mineral in the last three years 21 18. Mining leases marked on the Map of the District 22 Details of the area of where there is a cluster of Mining Leases 19. -

Coimbatore District Profile
COIMBATORE DISTRICT PROFILE 1. Name of the District : Coimbatore, TamilNadu 2. Geographical Position : North Latitude between 11o00’58” and 11o01’61” East Longitude between 76o58’16” and 76o09’71” 3. Total Geographical area : 3670 Sq.km 4. District Headquarters name : Coimbatore (Source: Google map) 5. Demographic details S. No Particulars Value i) Population Male 1482228 Female 1434392 Total Population 2916620 Rural 854489 Urban 2062131 ii) Population density / Sq. km 601 iii) Literates Male (%) 1140737 (77%) Female (%) 915640 (64%) Total (%) 2056377 (71%) iv) Details on SC/ST population Male 327791 Female 230936 Total Population 558727 Literacy rate (%) Male NA Female NA Total NA v) Labour profile a) Total workers 937314 b) Male workers 627693 c) Female workers 309621 d) Rural workers 436831 e) Urban workers 500483 f) Cultivators 98364 g) Agricultural Labourers 211056 h) Household industry 30381 i) Other workers 510302 j) Marginal workers 87211 j) Non-workers 1048424 k) Average labour wages for farm operations Skilled job Unskilled (Rs./manday of seven to nine hours) job Peak seasons NA Male NA Female NA Lean seasons Male NA Female NA Average labour wages for farm operations Skilled job Unskilled (Rs./manday of five to six hours) job Peak seasons Male 122.70 114.11 Female 64. 81 51.52 Lean seasons Male 114.11 95.62 Female 51.52 49.78 vi) Major languages spoken in the district Tamil, Telugu, Kannada vii) Details on Birth-Death Rate (per 1000 population) Birth Rate 15.9 Death Rate 5.4 Infant Mortality Rate 16.5 Expectation of life in years Male 65 Female 62 (Source : G returns and seasonal report) 6. -

Download the PDF a Megalithic Pottery Inscription and A
$0HJDOLWKLF3RWWHU\,QVFULSWLRQDQGD+DUDSSD 7DEOHW$FDVHRIH[WUDRUGLQDU\UHVHPEODQFH IRAVATHAM MAHADEVAN 1. Introduction The purpose of this paper is to bring to the notice of scholars a case of extraordinary resemblance between a megalithic pottery inscription of ca. first century BCE found at Sulur, near Coimbatore in Tamilnadu, South India, (and now in the British Museum, London), and a near-identical inscription on a miniature tablet from Harappa (and now in the collections of the Archaeological Survey of India, New Delhi). I am grateful to Dr.J.Robert Knox, formerly Keeper, Department of Oriental Antiquities in the British Museum, for the excellent photograph of the Sulur Dish (Fig.1). I also acknowledge my indebtness to Asko Parpola and the co-authors of the Corpus of Indus Seals and Inscriptions, vol. I, for the clear photograph of the miniature tablet from Harappa (Fig.2). Both objects have been published earlier; but the comparison between the two inscriptions is attempted here for the first time. Some of the material in the present paper is taken from my earlier paper on the Sulur Dish (Mahadevan 2001) with some modifications on the basis of fresh appraisal. Indus sign numbers in two or three digits and four-digit Indus text numbers are from my book (Mahadevan 1977). 2. The Sulur Dish (Fig.1 and detail in Fig.1A) Sulur is a well-known ancient site in Tamilnadu, which has yielded several antiquities including semi-precious stone beads, rouletted ware, punch- marked and Roman coins, assigned to the Late Megalithic-Iron Age and Early Historical Periods (Beck 1930; K.R.Srinivasan & N.R.Banerjee 1953; S.Suresh 2004). -
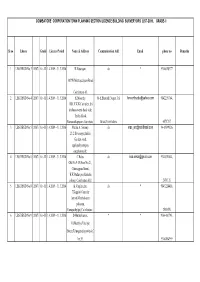
Sl No Lbs No Grade Licence Period Name & Address Communication
COIMBATORE CORPORATION TOWN PLANNING SECTION /LICENCE BUILDING SURVERYORS LIST-2009, GRADE-II Sl no Lbs no Grade Licence Period Name & Address Communication Add Email phone no Remarks 1 LBS REGD No 3 /2007/ Gr - II 1.4.2009 - 31.3.2010 R.Natarajan, do * 9360658377 34/270,Mettupalayam Road, Coimbatore.43. 2 LBS REGD No 4 /2007/ Gr - II 1.4.2009 - 31.3.2010 K.Moorthy, 56-E,Bharathi Nagar, 3rd [email protected] 9842239764, 1681.V.K.K.Complex, Sri kirshna sweets back side, Trichy Road, Ramanathapuram.Coimbator Street,Coimbatore 6571767 3 LBS REGD No 5 /2007/ Gr - II 1.4.2009 - 31.3.2010 Muthu A. Swamy, do [email protected] 9443899126 21/2 Siva nagar,Indira Garden road, upplipalayam(po), coimbatore.015 4 LBS REGD No 6 /2007/ Gr - II 1.4.2009 - 31.3.2010 C.Babu, do [email protected] 9344830644, Old No.9/15,New No.21,, Chinnappan Street, K.K.Pudur(po),Saibaba colony, Coimbatore.038 2451120 5 LBS REGD No 8 /2007/ Gr - II 1.4.2009 - 31.3.2010 K.Vanjimuthu, do * 9843224410, 7,Kappini Gounder layout,Maniyakaran palayam, Ganapathy(po),Coimbatore.6 2538470 6 LBS REGD No 9 /2007/ Gr - II 1.4.2009 - 31.3.2010 D.Muthu kumar, * * 9364410793, 10,Muthiya Udaiyar Street,Telungupalayam(po),C be.39. 9360804959 7 LBS REGD No 10 /2007/ Gr - II 1.4.2009 - 31.3.2010 V.Rangasamy, do [email protected] 9843623411 36/21,Supraya Mudaliar Street, Konavaikkal Palayam, Podanur(po).Coimbatore.023 . 8 LBS REGD No 11 /2007/ Gr - II 1.4.2009 - 31.3.2010 R.Subramaniam, do * 9345960702 1524,Avanashi road, Oppo.Sri Varadaraja Mills, Peelamedu, Coimbatore.4. -

Piscine Diversity of Coimbatore Wetlands, Tamilnadu, India
International Journal of Fisheries and Aquatic Studies 2016; 4(4): 280-285 ISSN: 2347-5129 (ICV-Poland) Impact Value: 5.62 (GIF) Impact Factor: 0.352 Piscine diversity of Coimbatore wetlands, Tamilnadu, IJFAS 2016; 4(4): 280-285 © 2016 IJFAS India www.fisheriesjournal.com Received: 05-05-2016 Accepted: 06-06-2016 Priyatharasini P and Dr. B Dhanalakshmi Priyatharasini P Abstract Research Scholar, PG and Wetlands of India preserve a rich variety of fish species.Globally wetlands as well as fauna and flora Research Department of diversity are affected due to increase in anthropogenic activities. The present investigation deals with the Zoology, Nirmala College for fish diversity of selected major wetlands Periyakulam famously called Ukkadam Lake, Singanallur Lake Women, Coimbatore-18, Tamil and Sulur Lake of Coimbatore district fed by Noyyal River. Due to improper management of these lentic Nadu, India. wetlands water bodies around Coimbatore district by using certain manures, insecticides in agricultural Dr. B Dhanalakshmi practices in and around these selected areas has polluted the land and these fresh waters creating hazards Assistant Professor, PG and for major vertebrate fishes which are rich source of food and nutrition, an important and delicious food of Research Department of man. The results of the present investigation reveals the occurrence of 19 fish species belonging to 5 Zoology, Nirmala College for order, 8 families 18 species recorded from the Ukkadam wetland followed by Singanallur wetland with 5 Women, Coimbatore-18, Tamil different orders 7 different families and 14 species. Ichthyofaunal diversity of Sulur wetland compressed Nadu, India. of 6 families with 14 species. -

Reply Affidavit of the 6Th Respondent in OA 190
BEFORE THE HON'BLE NATIONAL GREEN TRBUNAL(SZ) OA No. 190 of 2020 C. Palanisamy, Coimbatore Applicant Vs Mr. Murugesan, Naveen Tex, Thottathu Salai 6th Street, Vagarayam Palayam, Sulur Taluk, Moppiripalayam, Coimbatore- 641 659. (Having Factory at S.F. No. 208/5, Mooppiripalayam Village) and others ..Respondents REPLY AFFIDAVIT OF 6th RESPONDENTI No Description Page No 1. Reply affidavit of the 6th Respondent 01 2. Copy of the RTI with reply translated copy 06 3. Photograph Copy 08 Certified that the above documents are true copies of their Dated at Chennai on this the 8th day of March, 2021 original. COUNSEL FOR 6th RESPONDENT BEFORE THE HON'BLE NATIONAL GREEN TRIBUNAL(SZ) OA No. 190 of 2020 C. Palanisamy, Coimbatore Applicant Versus 1. The Chairman, Tamil Nadu Pollution Control Board, 76, Mount Road, Guindy, Chennai- 600 032 2. The District Collector, Coimbatore District, Coimbatore 3. The District Environmental Engineer, Coimbatore South, Tamil Nadu Pollution Control Board, Plot No. E-55A, SIDCO Industrial Estate, Kurichi, Pollachi Main Road, Coimbatore- 641 621 4. The Tahsildar, Sulur Taluk, Sulur, Coimbatore District. 5. The Executive Oficer, Moppiripalayam Town Panchayat Union Ofice, Vagarayampalayam, Coimbatore District- 641 659 6. Mr. Murugesan, Naveen Tex, Thottathu Salai 6th Street, Vagarayam Palayam, Sulur Taluk, Moppiripalayam, Coimbatore- 641 659. (Having Factory at S.F. No. 208/5, Mooppiripalayam Village) .. Respondents Reply Afidavit filed by 6th Respondent , Murugesan, S/o. Chinna Nanjappa Gounder, Hindu, aged about 50 years, residing at Thottathu Salai 6th Street, Vagarayam Palayam, Sulur Taluk, Moppiripalayam, Coimbatore- 641 659., now temporarily come down to do Chennai, hereby solemnly affirm and sincerely state as follows; I am the proprietor of the 6th Unit Respondent herein and as such, I am well acquainted with the facts and circumstances of the case. -

Tamil Nadu Government Gazette
© GOVERNMENT OF TAMIL NADU [Regd. No. TN/CCN/467/2009-11. 2009 [Price : Rs. 170. 40 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 28B] CHENNAI, WEDNESDAY, JULY 22, 2009 Aadi 6, Thiruvalluvar Aandu–2040 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU DENTAL COUNCIL NOTICE OF ELECTION TO THE DENTAL COUNCIL OF INDIA, NEW DELHI AND TO THE TAMIL NADU DENTAL COUNCIL, CHENNAI (Ref. No. TNDC/GCP/PER/09-2.) No. VI(1)/218/2009. Notice under Dental Council of India Election Regulations, 1952—Section 3(3) AND Notice under Tamil Nadu Dental Council Rules—G.O. No. 2638/Health and Family Welfare Department/Government of Tamil Nadu, dated 24-07-1950 — Election Rules — Section 2(3) Election of one member to the Dental Council of India under Section 3(a) AND Election of four members to the Tamil Nadu Dental Council under Section 21(a) AND Election of four members to the Tamil Nadu Dental Council under Section 21(b) of The Dentists Act, 1948. The Preliminary Electoral Roll as on 30th June 2009, showing the particulars of names of the registered Dentists to vote in the elections mentioned above, is published in the Tamil Nadu Government Gazette, Issue No. 28B, dated 22nd July 2009. Claims and Objections relating to the entries or omissions in the Preliminary Electoral Roll, with proof, shall be sent by registered post only to the undersigned on or before 31st August 2009 at 5 p.m. Claims and Objections received after the said date will not be accepted. -

List of Polling Stations for 116 Sulur Assembly Segment Within the 20 Coimbatore Parliamentary Constituency
List of Polling Stations for 116 Sulur Assembly Segment within the 20 Coimbatore Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 12 3 4 5 1 1 Panchayat Union Elementary 1.Pathuvampalli (R.V) and (P) Pathuvampalli Ward No.1 , 2.Pathuvampalli (R.V) All Voters School, Paduvampalli-641 659 and (P) Pathuvampalli Arijana Colony Ward No.1 , 3.Paduvampalli (R.V) and (P) Jangamanayakarpalayam Ward No.2 2 2 Panchayat Union Elementary 1.Pathuvampalli (R.V) and (P) Sennappachettipudur Ward No.5 , 2.Pathuvampalli All Voters School, Chennappachettipudur- (R.V)and (P) Channappachettiputhur Arijana Colony Ward No.5 , 3.Pathuvampalli 641 659 (R.V) and (P) Parakkadu Ward No.5 3 3 Panchayat Union Elementary 1.Paduvampalli (R.V) and (P) Sundamettupudhur Ward No.4 , 2.Paduvampalli All Voters School, Selambarayampalayam- (R.V) and (P) Selambarayampalayam Ward No.3 , 3.Paduvampalli (R.V) and (P) 641 659 Selambarayampalayam Arijana Colony Ward No.3 4 4 Panchayat Union Elementary 1.Pathuvampalli (R.V) and (P) Rayarpalayam Ward No.6 , 2.Pathuvampalli (R.V) All Voters School ,M.Rayarpalayam-641 659 and (P) Anna Nagar Arijana Colony Ward No.6 5 5 Panchayat Union Middle School, , 1.Kaduvettipalayam (R.V) and (P) Nallagoundenpalayam Ward No.1 , All Voters Nallagoundan palayam-641659 2.Kaduvettipalayam (R.V) and (P) Muthugoundenputhur Ward No.2 , 3.Kaduvettipalayam (R.V) and (P) Nallagoundenpalayam Arijana Colony Ward No.1 6 6 Panchayat