Agastache Rugosa Alleviates the Multi-Hit Effect on Hepatic Lipid Metabolism, Infammation and Oxidative Stress During Nonalcoholic Fatty Liver Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patchouli Essential Oil Extracted from Pogostemon Cablin (Blanco) Benth
Advances in Environmental Biology, 8(7) May 2014, Pages: 2301-2309 AENSI Journals Advances in Environmental Biology ISSN-1995-0756 EISSN-1998-1066 Journal home page: http://www.aensiweb.com/aeb.html Characterization and Antimicrobial Activity of Patchouli Essential Oil Extracted From Pogostemon cablin [Blanco] Benth. [lamiaceae] Ahmad Karimi Ph.D. in pharmacy, University of Santo Tomas, Philippines ARTICLE INFO ABSTRACT Article history: The physico-chemical properties of Philippine patchouli oil, hydro-distilled from fresh Received 25 March 2014 leaves and young shoots of Pogostemon cablin were characterized and found to be Received in revised form 20 April within the specifications set by the United States Essential Oils Society. Philippine 2014 patchouli oil and commercial patchouli oil have the same major components as shown Accepted 15 May 2014 by GC-MS analyses: patchouli alcohol, d-guaiene, a-guaiene, a-patchoulene, Available online 10 June 2014 seychellene, [3-patchoulene, and transcaryophylene, with slightly lower concentrations in the Philippine oil. Using the disk diffusion method patchouli oil was found to be Key words: active against the gram-positive bacteria: Staphylococcus, Bacillus, and Streptococcus Pogostemon cablin, patchouli oil, species. Fifty five percent [11/20] of community and only 14.8% [9/61] of hospital- essential oil, antimierobial activity, Staphylococcus aureus isolates were susceptible to an MIC of 0.03% [v/v.] and Sixty- physico-chemical properties four percent or 23/36 of methicillin-resistant Staphylococcus aureus [MRSA] isolates was sensitive to patchouli oil at 0.06%, as opposed to only 44% or 11/25 of the sensitive strains. Philippine patchouli essential oil was also active against several dermatophytes at 0.25%. -

Effect of Bioactive Compound of Aronia Melanocarpa on Cardiovascular System in Experimental Hypertension
Hindawi Oxidative Medicine and Cellular Longevity Volume 2017, Article ID 8156594, 8 pages https://doi.org/10.1155/2017/8156594 Research Article Effect of Bioactive Compound of Aronia melanocarpa on Cardiovascular System in Experimental Hypertension 1 1 1,2 1 Martina Cebova, Jana Klimentova, Pavol Janega, and Olga Pechanova 1Institute of Normal and Pathological Physiology, Slovak Academy of Sciences, Bratislava, Slovakia 2Department of Pathology, Faculty of Medicine, Comenius University, Bratislava, Slovakia Correspondence should be addressed to Martina Cebova; [email protected] Received 4 August 2017; Revised 10 October 2017; Accepted 18 October 2017; Published 30 November 2017 Academic Editor: Victor M. Victor Copyright © 2017 Martina Cebova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aronia melanocarpa has attracted scientific interest due to its dense contents of different polyphenols. We aimed to analyse effects of Aronia melanocarpa (AME) extract on blood pressure (BP), lipid peroxidation, cytokine level, total NOS activity in the left ventricle (LV), and aorta of L-NAME-induced hypertensive rats. 12-week-old male WKY rats were assigned to the control group and groups treated with AME extract (57.90 mg/kg/day), L-NAME (40 mg/kg/day), or combination of L-NAME (40 mg/kg/day) and AME (57.90 mg/kg/day) in tap water for 3 weeks. NOS activity, eNOS protein expression, and conjugated diene (CD) concentration were determined in the LV and aorta. After 3 weeks of L-NAME treatment, BP was increased by 28% and concomitant treatment with AME reduced it by 21%. -

TRP Mediation
molecules Review Remedia Sternutatoria over the Centuries: TRP Mediation Lujain Aloum 1 , Eman Alefishat 1,2,3 , Janah Shaya 4 and Georg A. Petroianu 1,* 1 Department of Pharmacology, College of Medicine and Health Sciences, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates; [email protected] (L.A.); Eman.alefi[email protected] (E.A.) 2 Center for Biotechnology, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates 3 Department of Biopharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, The University of Jordan, Amman 11941, Jordan 4 Pre-Medicine Bridge Program, College of Medicine and Health Sciences, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates; [email protected] * Correspondence: [email protected]; Tel.: +971-50-413-4525 Abstract: Sneezing (sternutatio) is a poorly understood polysynaptic physiologic reflex phenomenon. Sneezing has exerted a strange fascination on humans throughout history, and induced sneezing was widely used by physicians for therapeutic purposes, on the assumption that sneezing eliminates noxious factors from the body, mainly from the head. The present contribution examines the various mixtures used for inducing sneezes (remedia sternutatoria) over the centuries. The majority of the constituents of the sneeze-inducing remedies are modulators of transient receptor potential (TRP) channels. The TRP channel superfamily consists of large heterogeneous groups of channels that play numerous physiological roles such as thermosensation, chemosensation, osmosensation and mechanosensation. Sneezing is associated with the activation of the wasabi receptor, (TRPA1), typical ligand is allyl isothiocyanate and the hot chili pepper receptor, (TRPV1), typical agonist is capsaicin, in the vagal sensory nerve terminals, activated by noxious stimulants. -

Effects of Fertilization and Drying Conditions on the Quality of Selected Chinese Medicinal Plants
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses October 2019 EFFECTS OF FERTILIZATION AND DRYING CONDITIONS ON THE QUALITY OF SELECTED CHINESE MEDICINAL PLANTS Zoe Gardner University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Agriculture Commons Recommended Citation Gardner, Zoe, "EFFECTS OF FERTILIZATION AND DRYING CONDITIONS ON THE QUALITY OF SELECTED CHINESE MEDICINAL PLANTS" (2019). Doctoral Dissertations. 1726. https://doi.org/10.7275/14298230 https://scholarworks.umass.edu/dissertations_2/1726 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. EFFECTS OF FERTILIZATION AND DRYING CONDITIONS ON THE QUALITY OF SELECTED CHINESE MEDICINAL PLANTS A Dissertation Presented by ZOË E. GARDNER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY SEPTEMBER 2019 Plant and Soil Sciences © Copyright by Zoë E. Gardner 2019 All Rights Reserved EFFECTS OF FERTILIZATION AND DRYING CONDITIONS ON THE QUALITY OF SELECTED CHINESE MEDICINAL PLANTS A Dissertation Presented by ZOË E. GARDNER Approved as to style and content by: ____________________________________ Lyle E. Craker, Chair ____________________________________ Masoud Hashemi, Member ____________________________________ Michael R. Sutherland, Member __________________________________ Wesley Autio, Department Head Plant and Soil Sciences ACKNOWLEDGMENTS I am grateful to the incredible community of people that have helped in so many different ways to complete this research. -

In Vitro Antibacterial Activity of 34 Plant Essential Oils Against Alternaria Alternata
E3S Web o f Conferences 136, 06006 (2019) https://doi.org/10.1051/e3sconf/20191360 6006 ICBTE 2019 In vitro antibacterial activity of 34 plant essential oils against Alternaria alternata Qiyu Lu1, Ji Liu2, Caihong Tu2, Juan Li3, Chunlong Lei3, Qiliang Guo2, Zhengzhou Zhang2, Wen Qin1* 1College of Food Science and Technology, Sichuan Agricultural University, Ya'an, Sichuan, 625014, China 2Institute of Food Science and Technology, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China 3 Institute of Animal Science, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China Abstract. To determine the antibacterial effect of 34 plant essential oils on Alternaria alternata, 34 plant essential oils such as asarum essential oil, garlic essential oil, and mustard essential oil are used as inhibition agents to isolate A. alternata from citrus as indicator bacteria, through the bacteriostasis test and drug susceptibility test, the types of essential oils with the best inhibitory effect were screened and their concentration was determined. The results showed that the best inhibition effect was mustard essential oil with a minimum inhibitory concentration of 250 μl/L and a minimum bactericidal concentration of 250 μl/L. Followed by the Litsea cubeba essential oil and basil oil, the minimum inhibitory concentration is 500 μl/L. 1 Introduction quality and safety. A. alternata is the main fungus causing post-harvest disease of Pitaya, and the Citrus is a general term for orange, mandarin, orange, antibacterial effect of using cinnamon oil and clove oil is kumquat, pomelo, and medlar. Citrus is the world's good [10]. Clove oil can effectively inhibit the growth of largest fruit, rich in sugar, organic acids, mineral A. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Anti‑Inflammatory and Antioxidant Activity of the Traditional Herbal
MOLECULAR MEDICINE REPORTS 13: 4365-4371, 2016 Anti‑inflammatory and antioxidant activity of the traditional herbal formula Gwakhyangjeonggi‑san via enhancement of heme oxygenase‑1 expression in RAW264.7 macrophages SOO-JIN JEONG1,2, OHN-SOON KIM1, SAE-ROM YOO3, CHANG-SEOB SEO3, YEJI KIM3,4 and HYEUN-KYOO SHIN3 1KM Convergence Research Division, Korea Institute of Oriental Medicine, Daejeon 34054; 2Korean Medicine Life Science, University of Science and Technology, Yuseong-gu, Daejeon 34113; 3K-herb Research Center, Korea Institute of Oriental Medicine, Daejeon, Chungcheong 34054; 4Mucosal Immunology Laboratory, Department of Convergence Medicine, University of Ulsan College of Medicine/Asan Medical Center, Seoul 05505, Republic of Korea Received April 8, 2015; Accepted December 21, 2015 DOI: 10.3892/mmr.2016.5084 Abstract. Gwakhyangjeonggi-san (GHJGS) is a mixture of Introduction herbal plants, including Agastache rugosa, Perilla frute‑ scens, Angelica dahurica, Areca catechu, Poria cocos, Gwakhyangjeonggi-san (GHJGS) is a traditional Korean Magnolia officinalis, Atractylodes macrocephala, herbal formula composed of the following 13 medicinal herbs, Citrus reticulata, Pinellia ternata, Platycodon grandiflorum, Agastache rugosa, Perilla frutescens, Angelica dahurica, Glycyrrhiza uralensis, Ziziphus jujuba and Zingiber offici‑ Areca catechu, Poria cocos, Magnolia officinalis, nale. GHJGS has been used for treating diarrhea-predominant Atractylodes macrocephala, Citrus reticulata, Pinellia ternata, irritable bowel syndrome in traditional -

Pharmacological Effects of Agastache Rugosa Against Gastritis Using A
biomolecules Article Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach Hyeon-Hwa Nam, Joong Sun Kim , Jun Lee , Young Hye Seo, Hyo Seon Kim, Seung Mok Ryu, Goya Choi , Byeong Cheol Moon and A Yeong Lee * Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine, 111 Geonjae-ro, Jeollanam-do 58245, Korea; [email protected] (H.-H.N.); [email protected] (J.S.K.); [email protected] (J.L.); [email protected] (Y.H.S.); [email protected] (H.S.K.); [email protected] (S.M.R.); [email protected] (G.C.); [email protected] (B.C.M.) * Correspondence: [email protected]; Tel.: +82-61-338-7128; Fax: +82-61-338-7136 Received: 22 July 2020; Accepted: 7 September 2020; Published: 9 September 2020 Abstract: Agastache rugosa is used as a Korean traditional medicine to treat gastric diseases. However, the active ingredients and pharmacological targets of A. rugosa are unknown. In this study, we aimed to reveal the pharmacological effects of A. rugosa on gastritis by combining a mice model and a network pharmacology method. The macrophage and gastritis-induced models were used to evaluate the pharmacological effects of A. rugosa. The results show that A. rugosa relieved mucosal damage induced by HCl/EtOH in vivo. Network analysis identified 99 components in A. rugosa; six components were selected through systematic screening, and five components were linked to 45 gastritis-related genes. The main components were acacetin and luteolin, and the identified core genes were AKT serine/threonine kinase 1 (AKT1), nuclear factor kappa B inhibitor alpha (NFKBIA), and mitogen-activated protein kinase-3 (MAPK3) etc. -

Development of Process for Purification of Α and Β-Vetivone from Vetiver Essential Oil & Investigation of Effects of Heavy
UNIVERSITY OF NEW SOUTH WALES PhD Thesis Proposal Development of process for purification of α and β-vetivone from Vetiver essential oil & Investigation of effects of heavy metals on quality and quantity of extracted Vetiver oil Thai Danh Luu {B. Agric. Sc. (Qld University, Australia), M. Sc. (VUB, Belgium)} School of Chemical Sciences and Engineering Unversity of New South Wales, Sydney 1 Supervisor: Prof Neil Foster Supervisor: Prof Neil Foster Co-supervisors: Prof Tam Tran 2 and Dr Paul Truong 3 1 School of Chemical Sciences and Engineering, UNSW 2 Division of Engineering, Science and Technology, UNSW-Asia 3 Veticon Consulting and International Vetiver Network, Brisbane 1 I. INTRODUCTION In recent years, there is an increasing trend in research of essential oils extracted from various herbs and aromatic plants due to the continuous discoveries of their multifunctional properties other than their classical roles as food additives and/or fragrances. New properties of many essential oils, such as antibacterial, antifungal, antioxidant, and anti-inflammatory activities have been found and confirmed (Aruoma et al, 1996; Hammer et al, 1999; Güllüce et al, 2003). The pharmacological properties of essential oils extracted from plants have received the greatest interest of academic institutes and pharmaceutical companies (Ryman, 1992; Loza- Tavera, 1999; Courreges et al, 2000; Carnesecchi et al, 2002 and Salim et al, 2003). On the other hand, the insecticidal activities of essential oils are more favored by agricultural scientists and agri-business. Consequently, many investigation and new findings have significantly prompted and expanded novel applications of essential oils which are now been widely used as natural insecticides, cosmeceuticals, and aroma therapeutic agents. -
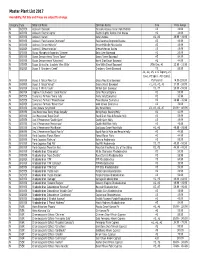
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

2019 Program WELCOME
THE SCOTT ARBORETUM OF SWARTHMORE COLLEGE www.scottarboretum.org 2019 Program WELCOME Welcome TABLE OF CONTENTS Greetings! Welcome to the 2019 Scott Arboretum Selections: Spring Sale. Download this handbook at scottarboretum.org. WELCOME 2 Schedule of the Sale 3 Special Offer Special Friends 4 10% discount on sales $100 and over, applies to plants only. Planting Container Grown Plants 10 Meaning of our Labels 12 Refund Policy Plant List 13 ALL SALES ARE FINAL; NO EXCHANGES OR REFUNDS. We are not able to offer refunds or exchanges since this is a special once-a- year event. Thank you! Many thanks to those volunteers who have contributed their efforts to this sale. A special thank you to Alan Kruza and Eve Thryum whose unwavering support and passion for the plants makes this sale possible. 2 SCHEDULE OF THE SALE Scott Arboretum Selections: Spring Sale Schedule: Friday, May 10 Special Friends Preview Party 5:30 to 7:30 pm To become a Special Friend to attend our Preview Party, call the Scott Arboretum Offices at 610- 328-8025. Saturday, May 11 Members Shopping 10 am – noon Members must show their membership card for early admission. If you have lost or misplaced your card, or would like to become a member, please call 610-328-8025. Open to the public – free noon – 3 pm 3 SPECIAL FRIENDS Julia and Vincent Auletta Our sincere appreciation to William D. Conwell Charles and Rosemary Philips these Special Friends of the Scott Laura Axel Arboretum Selections Sales, whose Harold Sweetman Alice Reilly support helps underwrite the cost of these vital fund-raising events. -

Relationships Between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties As a Tool for Botanical Discrimination
molecules Article Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination Monika K˛edzierska-Matysek 1, Małgorzata Stryjecka 2, Anna Teter 1 , Piotr Skałecki 1, Piotr Domaradzki 1 and Mariusz Florek 1,* 1 Institute of Quality Assessment and Processing of Animal Products, University of Life Sciences in Lublin, Akademicka 13, 20-950 Lublin, Poland; [email protected] (M.K.-M.); [email protected] (A.T.); [email protected] (P.S.); [email protected] (P.D.) 2 Institute of Agricultural Sciences, State School of Higher Education in Chełm, Pocztowa 54, 22-100 Chełm, Poland; [email protected] * Correspondence: mariusz.fl[email protected]; Tel.: +48-81-4456621 Abstract: The study compared the content of eight phenolic acids and four flavonoids and the antioxidant activity of six Polish varietal honeys. An attempt was also made to determine the correlations between the antioxidant parameters of the honeys and their polyphenol profile using principal component analysis. Total phenolic content (TPC), total flavonoid content (TFC), antioxidant activity (ABTS) and reduction capacity (FRAP) were determined spectrophotometrically, and the phenolic compounds were determined using high-performance liquid chromatography (HPLC). The buckwheat honeys showed the strongest antioxidant activity, most likely because they had the highest Citation: K˛edzierska-Matysek,M.; concentrations of total phenols, total flavonoids, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid, Stryjecka, M.; Teter, A.; Skałecki, P.; vanillic acid and chrysin. The principal component analysis (PCA) of the data showed significant Domaradzki, P.; Florek, M.