Mixed Evergreen Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Name Scientific Name Comments Evergreen Trees
Common Name Scientific Name Height* Spread Native Fall Color Ornamental Bark Flowering Wind Tolerant Tolerant Salt Well Drained Soil Moist Soil Full Sun Partial Sun Shade Comments Evergreen Trees Austrian Pine Pinus nigra 60' 30' x x x Vigerous, dark green needles (Behind Brenner's Castle Hill parking lot) Blue Spruce Picea pungens 60 30 x x Slow growing, bluish tint to needles English Laurel Prunus laurocerasus 10' 15' x x x x x x Good hedge.Dark green waxy leaves. (Corner Observatory & Seward St) Holly Ilex species 10' 10' x x Beautiful foliage and berries. Need male & female (City Hall west side) Lodgepole Pine (Bull Pine) Pinus contorta 35' 35' x x x x x x Fast growing, good for containers, screening (Crescent Park by picnic shelters) Mountain Hemlock Tsuga mertensiana 30' 15' x x x x x x x x Good for slopes, rock gardens, containers. Slow growing. (Wells Fargo parking lot) Sitka Spruce Picea sitchensis 100' 50' x x x x x Prone to aphids. Prolific, native of SE Alaska Western Hemlock Tsuga heterophylla 100 50 x x x x x x Fast growing. Can be pruned into a hedge (SJ Campus -Jeff Davis St.) Western Red Cedar Thuja plicata 80' 40' x x Beautiful foliage. Interesting bark. Subalpine Fir Abies lasiocarpa 100' 20' x Beautiful conical form. (Two across from Market Center parking lot.) Noble Fir Abies procera 100' 30' x Dark green, fast growing, beautiful large specimens at 1111 HPR Siberian Spruce Picea Omorika 20' 4' x x x Blue-green foliage. 'Bruns' at Moller Field, 'Weeping Brun's' at BIHA office Japanese White Pine Pinus parviflora 6' 3' x x Negishi' at Moller Field with blue-green foliage Korean Fir Abies koreana 15' 10' x x Horstmann's silberlocke' at Moller Field; silver foliage Western White Pine Pinus monticola 60' 20' x x x Fast growing, conical form (Fine Arts Camp Rasmusen Center, Lake St. -

The Mediterranean Forests Are Extraordinarily Beautiful, a Fascinating an Extraordinary Patrimony of Wealth Whose Conservation Can Be Highly Controversy
THE editerraneanFORESTS mA NEW CONSERVATION STRATEGY 1 3 2 4 5 6 the unveiled a meeting point the mediterranean: amazing plant an unknown millennia forests on the global 200 the terrestrial current a brand new the state of WWF a new approach wealth of the of nature a sea of forests diversity animal world of human the wane in the sub-ecoregions mediterranean tool: the gap mediterranean in action for forest mediterranean and civilisations interaction with mediterranean in the forest cover analysis forests protection forests forests mediterranean 23 46 81012141617 18 19 22 24 7 1 Argania spinosa fruits, Essaouira, Morocco. Credit: WWF/P. Regato 2 Reed-parasol maker, Tunisia. Credit: WWF-Canon/M. Gunther 3 Black-shouldered Kite. Credit: Francisco Márquez 4 Endemic mountain Aquilegia, Corsica. Credit: WWF/P. Regato 5 Sacred ibis. Credit: Alessandro Re 6 Joiner, Kure Mountains, Turkey. Credit: WWF/P. Regato 7 Barbary ape, Morocco. Credit: A. & J. Visage/Panda Photo It is like no other region on Earth. Exotic, diverse, roamed by mythical WWF Mediterranean Programme Office launched its campaign in 1999 creatures, deeply shaped by thousands of years of human intervention, the to protect 10 outstanding forest sites among the 300 identified through cradle of civilisations. a comprehensive study all over the region. When we talk about the Mediterranean region, you could be forgiven for The campaign has produced encouraging results in countries such as Spain, thinking of azure seas and golden beaches, sun and sand, a holidaymaker’s Turkey, Croatia and Lebanon. NATURE AND CULTURE, of forest environments in the region. But in recent times, the balance AN INTIMATE RELATIONSHIP Long periods of considerable forest between nature and humankind has paradise. -

EVERGREEN TREES for NEBRASKA Justin Evertson & Bob Henrickson
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS EVERGREEN TREES FOR NEBRASKA Justin Evertson & Bob Henrickson. For more plant information, visit plantnebraska.org or retreenbraska.unl.edu Throughout much of the Great Plains, just a handful of species make up the majority of evergreens being planted. This makes them extremely vulnerable to challenges brought on by insects, extremes of weather, and diseases. Utilizing a variety of evergreen species results in a more diverse and resilient landscape that is more likely to survive whatever challenges come along. Geographic Adaptability: An E indicates plants suitable primarily to the Eastern half of the state while a W indicates plants that prefer the more arid environment of western Nebraska. All others are considered to be adaptable to most of Nebraska. Size Range: Expected average mature height x spread for Nebraska. Common & Proven Evergreen Trees 1. Arborvitae, Eastern ‐ Thuja occidentalis (E; narrow habit; vertically layered foliage; can be prone to ice storm damage; 20‐25’x 5‐15’; cultivars include ‘Techny’ and ‘Hetz Wintergreen’) 2. Arborvitae, Western ‐ Thuja plicata (E; similar to eastern Arborvitae but not as hardy; 25‐40’x 10‐20; ‘Green Giant’ is a common, fast growing hybrid growing to 60’ tall) 3. Douglasfir (Rocky Mountain) ‐ Pseudotsuga menziesii var. glauca (soft blue‐green needles; cones have distinctive turkey‐foot bract; graceful habit; avoid open sites; 50’x 30’) 4. Fir, Balsam ‐ Abies balsamea (E; narrow habit; balsam fragrance; avoid open, windswept sites; 45’x 20’) 5. Fir, Canaan ‐ Abies balsamea var. phanerolepis (E; similar to balsam fir; common Christmas tree; becoming popular as a landscape tree; very graceful; 45’x 20’) 6. -

Global Ecological Forest Classification and Forest Protected Area Gap Analysis
United Nations Environment Programme World Conservation Monitoring Centre Global Ecological Forest Classification and Forest Protected Area Gap Analysis Analyses and recommendations in view of the 10% target for forest protection under the Convention on Biological Diversity (CBD) 2nd revised edition, January 2009 Global Ecological Forest Classification and Forest Protected Area Gap Analysis Analyses and recommendations in view of the 10% target for forest protection under the Convention on Biological Diversity (CBD) Report prepared by: United Nations Environment Programme World Conservation Monitoring Centre (UNEP-WCMC) World Wide Fund for Nature (WWF) Network World Resources Institute (WRI) Institute of Forest and Environmental Policy (IFP) University of Freiburg Freiburg University Press 2nd revised edition, January 2009 The United Nations Environment Programme World Conservation Monitoring Centre (UNEP- WCMC) is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme (UNEP), the world's foremost intergovernmental environmental organization. The Centre has been in operation since 1989, combining scientific research with practical policy advice. UNEP-WCMC provides objective, scientifically rigorous products and services to help decision makers recognize the value of biodiversity and apply this knowledge to all that they do. Its core business is managing data about ecosystems and biodiversity, interpreting and analysing that data to provide assessments and policy analysis, and making the results -

Resistance to Low and Negative Temperatures of Rhododendrons (Rhododendron) in the Botanical Garden of Šiauliai University in 2
Available online at www.notulaebotanicae.ro Print ISSN 0255-965X; Electronic ISSN 1842-4309 Not. Bot. Hort. Agrobot. Cluj 36 (1) 2008, 59-62 Notulae Botanicae Horti Agrobotanici Cluj-Napoca Resistance to Low and Negative Temperatures of Rhododendrons (Rhododendron) in the Botanical Garden of Šiauliai University in 2002-2007 Aurelija MALCIŪTĖ1) , Jonas Remigijus NAUJALIS2) , Antanas SVIRSKIS3) 1) Šiauliai University, Botanical Garden, Paitaičių Str. 4, LT-76284, Šiauliai, Lithuania, e-mail: [email protected] 2) Vilnius University, Faculty of Natural Sciences, Department of Botany and Genetics, M. K.Čiurlionio Str. 21/27, LT-03101, Vilnius, e-mail: [email protected] 3) Šiauliai University, Instituto 1, 58344 Akademija, Kėdainių distr., Lithuania, e-mail: [email protected] Abstract Rhododendrons are not native to Lithuania, but are often cultivated in botanical gardens, various public and private green plantations. Resistance to low temperatures are among the most important criteria in evaluating the condition of the rhododendron collection in the Botanical Garden of ŠU. The research initiated in the ŠU Botanical Garden will help in the selection and propagation of ornamental and tolerant to low temperatures representatives of species and cultivars, suitable for cultivation in northern Lithuania. Keywords: rhododendron, low temperature, ŠU Botanical Garden Introduction research on the plants of the Rhododendron genus in the region of Žemaitija uplands has been performed yet. Introduction of plants is one of the most important tasks of Botanical Garden of Šiauliai University (further Materials and methods referred as ŠU) which constantly expands the assortment of woody plants and that is the reason for the beginning Resistance to low temperatures are among the most of introduction of the Rhododendrons (Rhododendron L.) important criterion evaluated condition of rhododendrons genus plants. -

Using Evergreen Trees and Shrubs
Taking Another Look . Using Evergreen Trees, Shrubs and Ground Covers to Add Year-Round Interest to Your Landscape When most people hear the word evergreen, they think They come in all shapes — flat, horizontal, pyramid, of a Christmas tree. However, evergreen really refers to rounded, narrow, broad, irregular, and even contorted. any plant — a tree, a shrub or ground cover — that And if you don’t like a plant’s natural shape, there’s keeps the color in its needles or leaves all year long. always pruning. It’s easy to see where the name evergreen comes from! In contrast, deciduous plants lose their leaves once a Foliage color ranges from all shades of green, year in the fall and survive winters by becoming dormant gray-green and blue-green, as well as gold, yellow, (inactive) until spring — like maple trees, for example. cream, purple and red. Check out the new growth on evergreen plants in the springtime. The light shades of Whether considering evergreen trees, shrubs or ground new growth contrasted against the darker older foliage covers, evergreen plants can be divided into two broad are beautiful. categories: 3. Protection for birds. Evergreen trees, such as pines, spruces, and junipers, provide the essential hiding places that birds need for building nests and for shelter from Evergreens with needles winter winds. Common examples: Pine, spruce, juniper, fir and yew 4. Low maintenance and longevity. Once established, many evergreen plants require little maintenance and flourish for years. Evergreens with leaves Called broad-leaved evergreens Have you thought about these ways to use Common examples: Boxwood, evergreen plants? holly, azaleas, rhododendrons, euonymus and English ivy Accent and contrast. -

The Laurel Or Bay Forests of the Canary Islands
California Avocado Society 1989 Yearbook 73:145-147 The Laurel or Bay Forests of the Canary Islands C.A. Schroeder Department of Biology, University of California, Los Angeles The Canary Islands, located about 800 miles southwest of the Strait of Gibraltar, are operated under the government of Spain. There are five islands which extend from 15 °W to 18 °W longitude and between 29° and 28° North latitude. The climate is Mediterranean. The two eastern islands, Fuerteventura and Lanzarote, are affected by the Sahara Desert of the mainland; hence they are relatively barren and very arid. The northeast trade winds bring moisture from the sea to the western islands of Hierro, Gomera, La Palma, Tenerife, and Gran Canaria. The southern ends of these islands are in rain shadows, hence are dry, while the northern parts support a more luxuriant vegetation. The Canary Islands were prominent in the explorations of Christopher Columbus, who stopped at Gomera to outfit and supply his fleet prior to sailing "off the map" to the New World in 1492. Return voyages by Columbus and other early explorers were via the Canary Islands, hence many plants from the New World were first established at these points in the Old World. It is suspected that possibly some of the oldest potato germplasm still exists in these islands. Man exploited the islands by growing sugar cane, and later Mesembryanthemum crystallinum for the extraction of soda, cochineal cactus, tomatoes, potatoes, and finally bananas. The banana industry is slowly giving way to floral crops such as strelitzia, carnations, chrysanthemums, and several new exotic fruits such as avocado, mango, papaya, and carambola. -

Fluorescent Band Pattern of Chromosomes in Pseudolarix Amabilis, Pinaceae
© 2015 The Japan Mendel Society Cytologia 80(2): 151–157 Fluorescent Band Pattern of Chromosomes in Pseudolarix amabilis, Pinaceae Masahiro Hizume* Faculty of Education, Ehime University, 3 Bunkyo-cho, Matsuyama, Ehime 790–8577, Japan Received October 27, 2014; accepted November 18, 2014 Summary Pseudolarix amabilis belongs to one of three monotypic genera in Pinaceae. This species had 2n=44 chromosomes in somatic cells and its karyotype was composed of four long submetacentric chromosomes and 40 short telocentric chromosomes that varied gradually in length, supporting previous reports by conventional staining. The chromosomes were stained sequentially with the fluorochromes, chromomycin A3 (CMA) and 4′,6-diamidino-2-phenylindole (DAPI). CMA- bands appeared on 12 chromosomes at near terminal region and proximal region. DAPI-bands appeared at centromeric terminal regions of all 40 telocentric chromosomes. The fluorescent-banded karyotype of this species was compared with those of other Pinaceae genera considering taxonomical treatment and molecular phylogenetic analyses reported. On the basis of the fluorescent-banded karyotype, the relationship between Pseudolarix amabilis and other Pinaceae genera was discussed. Key words Chromomycin, Chromosome, DAPI, Fluorescent banding, Pinaceae, Pseudolarix amabilis. In Pinaceae, 11 genera with about 220 species are distinguished and grow mostly in the Northern Hemisphere (Farjon 1990). Most genera are evergreen trees, and only Larix and Pseudolarix are deciduous. Pinus is the largest genus in species number, and Cathaya, Nothotsuga and Pseudolarix are monotypic genera. The taxonomy of Pinaceae with 11 genera is complicated, having some problems in species or variety level. Several higher taxonomic treatments were reported on the base of anatomy and morphology such as resin canal in the vascular cylinder, seed scale, position of mature cones, male strobili in clusters from a single bud, and molecular characters in base sequences of several DNA regions. -

3. Canary Islands and the Laurel Forest 13
The Laurel Forest An Example for Biodiversity Hotspots threatened by Human Impact and Global Change Dissertation 2014 Dissertation submitted to the Combined Faculties for the Natural Sciences and for Mathematics of the Ruperto–Carola–University of Heidelberg, Germany for the degree of Doctor of Natural Sciences presented by Dipl. biol. Anja Betzin born in Kassel, Hessen, Germany Oral examination date: 2 The Laurel Forest An Example for Biodiversity Hotspots threatened by Human Impact and Global Change Referees: Prof. Dr. Marcus A. Koch Prof. Dr. Claudia Erbar 3 Eidesstattliche Erklärung Hiermit erkläre ich, dass ich die vorgelegte Dissertation selbst verfasst und mich dabei keiner anderen als der von mir ausdrücklich bezeichneten Quellen und Hilfen bedient habe. Außerdem erkläre ich hiermit, dass ich an keiner anderen Stelle ein Prüfungsverfahren beantragt bzw. die Dissertation in dieser oder anderer Form bereits anderweitig als Prü- fungsarbeit verwendet oder einer anderen Fakultät als Dissertation vorgelegt habe. Heidelberg, den 23.01.2014 .............................................. Anja Betzin 4 Contents I. Summary 9 1. Abstract 10 2. Zusammenfassung 11 II. Introduction 12 3. Canary Islands and the Laurel Forest 13 4. Aims of this Study 20 5. Model Species: Laurus novocanariensis and Ixanthus viscosus 21 5.1. Laurus ...................................... 21 5.2. Ixanthus ..................................... 23 III. Material and Methods 24 6. Sampling 25 7. Laboratory Procedure 27 7.1. DNA Extraction . 27 7.2. AFLP Procedure . 27 7.3. Scoring . 29 7.4. High Resolution Melting . 30 8. Data Analysis 32 8.1. AFLP and HRM Data Analysis . 32 8.2. Hotspots — Diversity in Geographic Space . 34 8.3. Ecology — Ecological and Bioclimatic Analysis . -
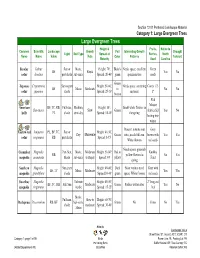
Category 1: Large Evergreen Trees
Section 12.01 Preferred Landscape Material Category 1: Large Evergreen Trees Large Evergreen Trees Height & Fruits, Native to Common Scientific Landscape Growth Fall Interesting Growth Drought Light Soil Type Spread at Berries, North Name Name Value Rate Color Patterns Tolerant Maturity Seed Carolina Deodar Cedrus Sun or Mesic, Height: 70'; Bluish- Needs space; excellent Green BF Rapid Yes No cedar deodara part shade sub-xeric Spread: 20-40' green specimen tree seeds Green Japanese Cryptomeria Sun to part Height: 50-60'; Needs space, screening Cones 1/2- BF Mesic Moderate to No No cedar japonica shade Spread: 25-30' material 1" bronze Red Mature American BF, TC, RB, Full-sun, Medium, Height: 50'; Small white flowers in Ilex opaca Slow Green fruit in fall Yes No holly PL shade semi-dry Spread: 18-40' the spring lasting into winter Doesn’t tolerate wet Gray Eastern red Juniperus PL, BF, TC, Sun or Height: 40-50'; Dry Moderate Green sites; needs full sun. brown with Yes Yes cedar virginiana RB part shade Spread: 8-15' White flowers red seeds Needs space. greenish Cucumber Magnolia Part-Sun, Mesic, Moderate Height: 50-80'; Red to Knobby RB yellow flowers in No Yes magnolia acuminata Shade sub-xeric to Rapid Spread: 40' yellow Fruit spring Southern Magnolia Sun, part Height: 40-80'; Dark Most varities need Gray with BF, TC Mesic Moderate Yes Yes magnolia grandiflora shade Spread:30-40' green space; White flowers red seeds Sweetbay Magnolia Tolerate Height: 40-50'; 2" long red BF, TC, RB Full Sun Moderate Green Prefers wetter sites Yes No magnolia -

Name That Gymnosperm
A B C D This tree is found frequently This evergreen is found throughout This species is found at drier and This Wyoming tree is a remnant of throughout the eastern half of the state. The species is known for lower elevations compared to some the last ice age and is found exclu- Wyoming ranging from the Black often having serotinous cones that of the other trees pictured. This tree sively in the Black Hills of Wyoming Hills to the Laramie Mountains and only open to release seeds when the shares its name with the town in and South Dakota. It grows at higher the Bighorns. The tree is known cone is heated. Once the limbs are central Wyoming. elevations along riparian or wet areas for its ability to withstand the heat removed, this tree makes an excel- in its native habitat. of wildfires because of its thick, lent pole for teepees because of its reddish-colored bark. long, slender trunk. NAME THAT GYMNOSPERM Wyoming is host to many conifer (gymnosperm or “naked in hot, dry, and low elevations while others are found in cold and seed” plants including conifer, cycads, and ginkos) tree species. high elevations. Cones are an excellent tool that can be used for The cones in this quiz and their parent trees are found at different identification of evergreens. Match the tree to the photo. Good geographical locations across the Cowboy State. Some are found luck and keep an eye out for these trees this year! E F G H Known for having extremely flexible This species is probably better known This tree is found in most high- This tree grows at high elevations limbs, this tree is often found brav- as an ornamental in Wyoming but is elevation forests of Wyoming. -

Gardenergardener®
Theh American A n GARDENERGARDENER® The Magazine of the AAmerican Horticultural Societyy January / February 2016 New Plants for 2016 Broadleaved Evergreens for Small Gardens The Dwarf Tomato Project Grow Your Own Gourmet Mushrooms contents Volume 95, Number 1 . January / February 2016 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2016 Seed Exchange catalog now available, upcoming travel destinations, registration open for America in Bloom beautifi cation contest, 70th annual Colonial Williamsburg Garden Symposium in April. 11 AHS MEMBERS MAKING A DIFFERENCE Dale Sievert. 40 HOMEGROWN HARVEST Love those leeks! page 400 42 GARDEN SOLUTIONS Understanding mycorrhizal fungi. BOOK REVIEWS page 18 44 The Seed Garden and Rescuing Eden. Special focus: Wild 12 NEW PLANTS FOR 2016 BY CHARLOTTE GERMANE gardening. From annuals and perennials to shrubs, vines, and vegetables, see which of this year’s introductions are worth trying in your garden. 46 GARDENER’S NOTEBOOK Link discovered between soil fungi and monarch 18 THE DWARF TOMATO PROJECT BY CRAIG LEHOULLIER butterfl y health, stinky A worldwide collaborative breeds diminutive plants that produce seeds trick dung beetles into dispersal role, regular-size, fl avorful tomatoes. Mt. Cuba tickseed trial results, researchers unravel how plants can survive extreme drought, grant for nascent public garden in 24 BEST SMALL BROADLEAVED EVERGREENS Delaware, Lady Bird Johnson Wildfl ower BY ANDREW BUNTING Center selects new president and CEO. These small to mid-size selections make a big impact in modest landscapes. 50 GREEN GARAGE Seed-starting products. 30 WEESIE SMITH BY ALLEN BUSH 52 TRAVELER’S GUIDE TO GARDENS Alabama gardener Weesie Smith championed pagepage 3030 Quarryhill Botanical Garden, California.