Multidisciplinary Consensus Statement on the Clinical Management of Patients with Stage III Non‑Small Cell Lung Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Airway Obstruction After Biopsy by Cervical Mediastinoscopy in a Patient with a Mediastinal Mass -A Case Report
Korean J Anesthesiol 2012 July 63(1): 65-67 Case Report http://dx.doi.org/10.4097/kjae.2012.63.1.65 Airway obstruction after biopsy by cervical mediastinoscopy in a patient with a mediastinal mass -A case report- Yong-Cheol Lee2, Sang-Jin Park1, and In-seong Kim1 Department of Anesthesiology and Pain Medicine, 1College of Medicine, Yeungnam University, 2School of Medicine, Keimyung University, Daegu, Korea Biopsy, using mediastinoscopy is commonly employed for accurate histologic diagnosis of a mediastinal mass. However, since the mass is not removed during the procedure, it may cause compression of vital structures such as major airways, the heart, the pulmonary artery, and the superior vena cava after surgery. We observed a case of a 66-year-old man with a mediastinal mass that caused severe airway obstruction during recovery from anesthesia following mediastinoscopic biopsy, probably caused by upper airway edema which seemed to originate from compression of the superior vena cava. Therefore, we suggest that unexpected airway obstruction in a patient with a mediastinal mass can be due to superior vena cava compression. (Korean J Anesthesiol 2012; 63: 65-67) Key Words: Airway obstruction, Mediastinoscopy, Superior vena cava. Biopsies, using mediastinoscopy are commonly utilized Case Report to decide on the treatment of mediastinal masses. However, since the mass is not removed during the biopsy, it may cause A 66-year-old man visited the hospital with a one month compression of vital structures after the procedure [1]. The history of chest discomfort and sporadic swelling in the face superior vena cava has an especially low intravascular pressure and arms in the morning. -

Update in Anaesthesia
Update in Anaesthesia Pulmonary Function Tests and Assessment for Lung Resection David Portch*, Bruce McCormick *Correspondence Email: [email protected] INTRODUCTION Summary respectively. There are 2400 lobectomies and 500 The aim of this article is to describe the tests available This article describes the for the assessment of patients presenting for lung pneumonectomies performed in the UK each year, steps taken to evaluate resection. The individual tests are explained and we with in-hospital mortality 2-4% for lobectomy and patients’ fitness for lung 4 describe how patients may progress through a series of 6-8% for pneumonectomy. resection surgery. Examples tests to identify those amenable to lung resection. Lung resection is most frequently performed to treat are used to demonstrate interpretation of these tests. Pulmonary function testing is a vital part of the non-small cell lung cancer. This major surgery places It is vital to use these tests in assessment process for thoracic surgery. However, large metabolic demands on patients, increasing conjunction with a thorough for other types of surgery there is no evidence postoperative oxygen consumption by up to 50%. history and examination that spirometry is more effective than history and Patients presenting for lung resection are often high in order to achieve an examination in predicting postoperative pulmonary risk due to a combination of their age (median age accurate assessment of each complications in patients with known chronic lung is 70 years)5 and co-morbidities. Since non-surgical patient’s level of function. conditions. Furthermore specific spirometric values mortality approaches 100%, a thorough assessment of Much of this assessment (e.g. -

Consider a Minor Surgery As a Major Surgery and Order Preoperative Tests Accordingly (I.E
ROUTINE PREOPERATIVE LAB TEST GUIDELINES For adult patients (≥ 16 years) undergoing elective surgery TESTS WITHIN 6 MONTHS OF SURGERY CLINICAL JUDGEMENT IS REQUIRED EXCLUSIONS are valid, provided there has been no interim change as additional tests may be appropriate for patients with this guideline does not apply to patients in the patient’s condition. complex or uncommon surgical or medical conditions. undergoing cardiac surgery or cesarean section. MINOR SURGERY MAJOR SURGERY Associated with an expected blood loss of Associated with an expected blood loss of >500mL, significant fluid shifts and typically, at least one night in <500mL, minimal fluid shifts and is typically hospital*. Includes laparoscopic surgery (except cholecystectomy and tubal ligation); open resection of organs; done on an ambulatory basis (day surgery/ large joint replacements; mastectomy with reconstruction; and spine, thoracic, vascular, or intracranial surgery. same day discharge)*. It includes cataract * If the surgery is typically ambulatory but the patient has a medical or social reason for overnight admission (i.e. OSA, no support surgery; breast surgery without reconstruction; at home), still consider the surgery minor in determining which lab tests to order. laparoscopic cholecystectomy and tubal ligation; and most cutaneous, superficial, All Major Age: 16-49 years old Age: 50+ years old endoscopic and arthroscopic procedures. or Order • add ECG for patients with DM, HTN, Renal, • add ECG + + – DO NOT ORDER PREOP TESTS CBC Cardiovascular or severe Respiratory disease. + + – • add Na , K , Cl , TCO2 including: chest x-rays, Na , K , Cl , TCO , + + – 2 Na K Cl TCO & Cr/ eGFR serum glucose, CBC, ECG, INR, urinalysis, • add , , , 2 for patients with • add Cr/ eGFR renal, liver or thyroid function tests in DM; HTN; Malnutrition; BMI > 40; Renal, Liver or asymptomatic** patients. -

Mediastinoscopy: a Clinical Evaluation of 400 Consecutive Cases
Thorax: first published as 10.1136/thx.24.5.585 on 1 September 1969. Downloaded from Thorax (1969), 24, 585. Mediastinoscopy: A clinical evaluation of 400 consecutive cases C. L. SARIN1 AND H. C. NOHL-OSER From the Thoracic Surgical Unit, Harefield Hospital, Harefield, Middlesex Mediastinoscopy was carried out in 400 cases, including 296 of bronchogenic carcinoma. At the time of presentation the new growth had already spread to involve the mediastinal lymph nodes in slightly more than 50% of these. The incidence of involvement was 76% in oat-cell and 35% in squamous-cell carcinoma. Non-resectability at thoracotomy was encountered in seven out of 120 patients. We advocate this procedure in every case of bronchogenic carcinoma which is considered operable on other counts. In patients in whom the mediastinal lymph nodes are invaded by growth we prefer radical radiotherapy to surgery, as the long-term survival of the two methods is comparable. This procedure may be the only source of positive histological proof of diagnosis, not only in carcinoma but in other types of intrathoracic disease. We believe that this procedure reduces the number of unnecessary exploratory thoracotomies. Carlens (1959) introduced diagnostic exploration sible. Biopsy in such cases can be obtained from tissues inside the thoracic inlet. in the of the superior mediastinum. The space explored just Bleeding, copyright. is part of the superior mediastinum which is presence of incipient or developed superior vena caval of the obstruction, or dense fibrosis of the pre-tracheal situated around tihe intrathoracic part fascia, can make the procedure difficult or impossible. -

Thoracic Surgery Institution: Nashville VA Medical Center & Duration: 6 Weeks Vanderbilt University Medical Center Supervising Physician: Eric L
Thoracic Surgery Institution: Nashville VA Medical Center & Duration: 6 weeks Vanderbilt University Medical Center Supervising Physician: Eric L. Grogan, M.D. Contact Information: 615-300-2900 Year of Training: PGY-4 Educational Objectives: During this rotation, the resident will better understand the pathophysiology of thoracic diseases including lung, esophagus, and chest wall diseases. The resident will identify the general risks and complications of thoracic surgery operations, and learn the preoperative and postoperative care of patients undergoing thoracic surgery operations Evaluation of the resident's understanding of the patient and disease process will be reviewed (in part) at the time of operation and through resident-faculty interaction. Feedback will be verbal and timely; residents are encouraged to establish a dialogue with the faculty to facilitate feedback. Residents are expected to notify Dr. Grogan and meet with him when starting the service. Other Comments and Responsibilities Daily rounds will include the General Care Wards and the Intensive Care Unit at the VA. Medical Knowledge and Patient Care: I. CHEST WALL A. Anatomy, Physiology and Embryology Learner Objectives: • Understands the anatomy and physiology of the cutaneous, muscular, and bony components of the chest wall and their anatomic and physiologic relationships to adjacent structures; • Knows various operative approaches to the chest wall. Clinical Skills: • Recognizes the normal and abnormal anatomy of the chest wall. B. Acquired Abnormalities and Neoplasms Learner Objectives: • Evaluates and diagnoses primary and metastatic chest wall tumors, knows their histologic appearance, and understands the indications for incisional versus excisional biopsy; • Knows the radiologic characteristics of tumors. Clinical Skills: • Performs a variety of surgical incisions to expose components of the chest wall and interior thoracic organs. -

Chapter I GENERAL CORRECT CODING POLICIES for NATIONAL CORRECT CODING INITIATIVE POLICY MANUAL for MEDICARE SERVICES
CHAP1-gencorrectcodingpolicies Revision Date: 1/1/2021 Chapter I GENERAL CORRECT CODING POLICIES FOR NATIONAL CORRECT CODING INITIATIVE POLICY MANUAL FOR MEDICARE SERVICES Current Procedural Terminology (CPT) codes, descriptions and other data only are copyright 2020 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association. Applicable FARS\DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors, prospective payment systems, and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for the data contained or not contained herein. Revision Date (Medicare): 1/1/2021 Table of Contents LIST OF ACRONYMS ............................................ I-2 Chapter I ................................................... I-4 General Correct Coding Policies ............................ I-4 A. Introduction ..........................................I-4 B. Coding Based on Standards of Medical/Surgical Practice I-8 C. Medical/Surgical Package .............................I-11 D. Evaluation & Management (E&M) Services ...............I-16 E. Modifiers and Modifier Indicators ....................I-18 F. Standard Preparation/Monitoring Services for Anesthesia .................................................. I-26 G. Anesthesia Service Included in the Surgical Procedure I-26 H. HCPCS/CPT -
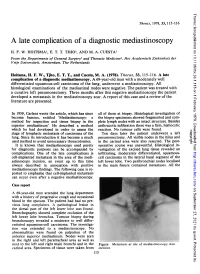
A Late Complication of a Diagnostic Mediastinoscopy
Thorax: first published as 10.1136/thx.33.1.115 on 1 February 1978. Downloaded from Thorax, 1978, 33, 115-116 A late complication of a diagnostic mediastinoscopy H. F. W. HOITSMA1, E. T. T. TJHO2, AND M. A. CUESTA' From the Departments of General Surgery' and Thoracic Medicine2, Het Academisch Ziekenhuis der Vrije Universiteit, Amsterdam, The Netherlands Hoitsma, H. F. W., Tjho, E. T. T., and Cuesta, M. A. (1978). Thorax, 33, 115-116. A late complication of a diagnostic mediastinoscopy. A 69-year-old man with a moderately well differentiated squamous-cell carcinoma of the lung, underwent a mediastinoscopy. All histological examinations of the mediastinal nodes were negative. The patient was treated with a curative left pneumonectomy. Three months after this negative mediastinoscopy the patient developed a metastasis in the mediastinoscopy scar. A report of this case and a review of the literature are presented. In 1959, Carlens wrote the article, which has since all of them at biopsy. Histological investigation of become famous, entitled 'Mediastinoscopy: a the biopsy specimens showed fragmented and com- method for inspection and tissue biopsy in the plete lymph nodes with an intact structure. Besides superior mediastinum'. He described a method anthracotic infiltration there was a firm, histiocytic which he had developed in order to assess the reaction. No tumour cells were found. copyright. stage of lymphatic metastasis of carcinoma of the Ten days later the patient underwent a left lung. Since its introduction it has become a much pneumonectomy. All visible nodes in the hilus and used method to avoid unnecessary thoracotomies. -

Cervical Mediastinal L SUSAN ALEXANDER L for STAGING of LUNG CANCER
Cervical Mediastinal l SUSAN ALEXANDER l FOR STAGING OF LUNG CANCER ervical mediastinal exp- bronchogenic lung cancer by sam- loration (CME), or pling selected lymph nodes in and mediastinoscopy, is a around the trachea, its major surgical procedure to bifurcation and the great vessels. C explore and sample Lymph nodes are removed and lymph nodes in the space between sent to pathology for tissue diag- the lungs, (the mediastinum), nosis to determine the histology when diagnostic imaging studies of the tumor. CME is performed (X-ray, CT scan, etc) suggest a primarily to stage lung cancer growth in the lungs or mediasti- and determine the extent of the nal region. The most common pur- disease and establish treatment pose of the CME is to diagnose options. DECEMBER 2002 The Surgical Technologist 9 224 DECEMBER 2002 CATEGORY 1 If cancer exists in the lymph nodes, the cell type nodes that are not accessible through CME. In (histology) identifies the type of cancer and one series of 100 patients with tumors in this extent of the lymph nodes involved. If tumor area, 22 were found to be inoperable despite hav involvement in the mediastinal area is demon ing a negative mediastinoscopy.5 Left anterior strated in the pathology review of the speci- mediastinotomy through the second intercostal men(s) (lymph nodes), the patient may be space is the preferred method to assess the oper spared an unnecessary thoracotomy; however, ability of these patients, as suggested by Pearson this means that the tumor is inoperable.5 and coworkers.5 Less than 50% of patients undergoing cura History tive resection for bronchogenic carcinoma sur CME was originally described by Harken and vive five years. -

Physicians As Assistants at Surgery: 2016 Update
Physicians as Assistants at Surgery: 2016 Update Participating Organizations: American College of Surgeons American Academy of Ophthalmology American Academy of Orthopaedic Surgeons American Academy of Otolaryngology – Head and Neck Surgery American Association of Neurological Surgeons American Pediatric Surgical Association American Society of Colon and Rectal Surgeons American Society of Plastic Surgeons American Society of Transplant Surgeons American Urological Association Congress of Neurological Surgeons Society for Surgical Oncology Society for Vascular Surgery Society of American Gastrointestinal Endoscopic Surgeons The American College of Obstetricians and Gynecologists The Society of Thoracic Surgeons Physicians as Assistants at Surgery: 2016 Update INTRODUCTION This is the seventh edition of Physicians as Assistants at Surgery, a study first undertaken in 1994 by the American College of Surgeons and other surgical specialty organizations. The study reviews all procedures listed in the “Surgery” section of the 2016 American Medical Association’s Current Procedural Terminology (CPT TM). Each organization was asked to review new codes since 2013 that are applicable to their specialty and determine whether the operation requires the use of a physician as an assistant at surgery: (1) almost always; (2) almost never; or (3) some of the time. The results of this study are presented in the accompanying report, which is in a table format. This table presents information about the need for a physician as an assistant at surgery. Also, please note that an indication that a physician would “almost never” be needed to assist at surgery for some procedures does NOT imply that a physician is never needed. The decision to request that a physician assist at surgery remains the responsibility of the primary surgeon and, when necessary, should be a payable service. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

A Novel Prediction Model for the Probability Of
Editorial Page 1 of 7 A novel prediction model for the probability of mediastinal lymph node metastases detected by endobronchial ultrasound-transbronchial needle aspiration in non-small cell lung cancer: possible applications in clinical decision-making Aldo Carnevale1, Gianluca Milanese2,3, Nicola Sverzellati2, Mario Silva2,3 1Section of Radiology, Department of Morphology Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy; 2Section of Radiology, Unit of Surgical Sciences, Department of Medicine and Surgery (DiMeC), University of Parma, Parma, Italy; 3bioMILD Lung Cancer Screening Trial, IRCCS Istituto Nazionale Tumori, Milan, Italy Correspondence to: Mario Silva. Section of Radiology, Pad. Barbieri, via Gramsci 14, 43126, Parma, Italy. Email: [email protected]. Comment on: O’Connell OJ, Almeida FA, Simoff MJ, et al. A Prediction Model to Help with the Assessment of Adenopathy in Lung Cancer: HAL. Am J Respir Crit Care Med 2017;195:1651-60. Received: 17 March 2018; Accepted: 08 May 2018; Published: 01 June 2018. doi: 10.21037/med.2018.05.02 View this article at: http://dx.doi.org/10.21037/med.2018.05.02 Surgery is the most successful radical approach to non-small and address the most appropriate therapy (e.g., surgery, cell lung cancer (NSCLC), provided accurate preoperative radiation therapy, or medical treatment) (6). systemic and mediastinal staging for stratification of patients Each of CT and PET show limitations in N staging, with potentially resectable disease (1). In particular, pre- especially with smaller lymph nodes (7). As a consequence, surgical mediastinal nodal staging (N staging) is performed the American College of Chest Physicians (ACCP) issued by a combination of diagnostic tests with different levels of guidelines with four main categories on the basis of thoracic accuracy and invasiveness (2,3), as well as cumulative costs radiographic appearance to drive the most appropriate use of and risks. -

Bronchoscopic Needle Aspiration in the Diagnosis of Mediastinal Lymphadenopathy and Staging of Lung Cancer
Free full text available from Review Article www.cancerjournal.net Bronchoscopic needle aspiration in the diagnosis of mediastinal lymphadenopathy and staging of lung cancer ABSTRACT Vikas Punamiya1, 2 Transbronchial needle aspiration (TBNA) has the potential to allow adequate mediastinal staging of non-small cell lung cancer with Ankur Mehta , Prashant N. Chhajed1,2 enlarged lymph nodes in most patients without the need for mediastinoscopy. Metastasis to the mediastinal lymph nodes is one of the most important factors in determining resectability and prognosis in non-small cell lung cancer. The importance of TBNA as a tool for 1Institute of Pulmonology, diagnosing intrathoracic lymphadenopathy as well as in the staging of lung cancer has been reported in various studies. We performed Medical Research & Development, Mumbai, a literature search in PubMed and Journal of Bronchology using the keyword transbronchial needle aspiration. TBNA is a safe and 2Fortis Hiranandani effective procedure to diagnose mediastinal lymphadenopathy. Real-time bronchoscopic ultrasound-guided TBNA is the new kid on Hospitals, Mumbai, India the block, which can further enhance the sensitivity of bronchoscopy in the diagnosis of mediastinal lesions. For correspondence: Dr. Prashant N. Chhajed, B/24, Datta Apartments, KEY WORDS: Bronchoscopy, lung cancer, mediastinoscopy, transbronchial needle aspiration Ramkrishna Mission Marg, 15th Road, Khar - West, Mumbai - 400 052, INTRODUCTION Transbronchial needle aspiration (TBNA) can be India. E-mail: pchhajed@gmail. performed at bronchoscopy to sample mediastinal com Differential diagnosis of mediastinal and hilar lymph nodes or mass lesions. We DOI: 10.4103/0973- lymphadenopathy ranges from lymph node performed a literature search in PubMed using 1482.65231 metastases, malignant lymphomas, infectious, the keyword transbronchial needle aspiration.