Research Journal of Pharmaceutical, Biological and Chemical Sciences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Handbook of Statistics 2014 Chittoor District Andhra Pradesh.Pdf
HAND BOOK OF STATISTICS CHITTOOR DISTRICT 2014 ***** Compiled and Published by CHIEF PLANNING OFFICER CHITTOOR DISTRICT SIDDHARTH JAIN I.A.S., District Collector & Magistrate, Chittoor District. PREFACE I am happy to release the Twenty EighthEdition of Hand Book of Statistics of Chittoor District which incorporates Statistical Data of various departments for the Year 2014. The Statistical Data in respect of various departments and schemes being implemented in the district are compiled in a systematic and scientific manner reflects the progress during the year. The sector-wise progress is given in a nutshell under the chapter “DISTRICT AT A GLANCE” apart from Mandal-wise data. The publication reflects the latest data on various aspects of the District Economy. The information has been given Mandal-wise in a concise form to facilitate an over all assessment of the District Economy for the year. This compilation will serve as a useful reference book for the General public, Planners, Administrators, Research Scholars, Bankers and also special Agencies that are involved in the formulation and implementation of various developmental programmes in the district. I am thankful to all District Officers and the heads of other institutions for extending their helping hand by furnishing their respective Statistical data to theChief Planning Officer for publication of this Hand Book. I appreciate the efforts made by Sri. Ch. V.S.BhaskaraSarma, Chief Planning Officer, Chittoor, other Officers and Staff Members of the Chief Planning Office in bringing out this publication which projects the development of the District during the year 2014. Any suggestions aimed at improving the quality of data incorporated in this Hand Book are most welcome. -

Data Base of Chittoor District
DATA BASE OF CHITTOOR DISTRICT. The district is categorized under Southern Agro Climatic Zone of Andhra Pradesh based on soil type, rainfall and altitude. There are 66 mandals, 1540 revenue villages and 1394 Panchayats in the district. In dry farming tracts of the zone groundnut is the main crop where as under tanks, wells and bore wells double cropping is practiced with Rice. After Groundnut and Paddy, Sugarcane occupies 3rd place in Chittoor District. At present year, the area under maize and Sunflower is increasing gradually in the district. Information is being collected regularly pertaining to Area, Production and Productivity of Agriculture, Horticulture, Sericulture, Animal husbandry and other related disciplines, updated and computerized systematically CHITTOOR DISTRICT Agricultural lands of the district comprise Red Soils - 57% Sandy loams - 34% Mixed Soils - 9% LAND UTILIZATION PATTERN IN THE DISTRICT (Area in ha) S. Particulars Area No. 1. Forest 4,51,345 2. Barren & Uncultivable land 1,64,265 3. Land Put to Non-Agril. Uses 1,57,000 4. Permanent Pastures & Other grazing lands 36,521 5. Miscellaneous tree crops & Groves not included in net area sown. 25,173 6. Cultivable waste 39,512 7. Other fallow lands 1,26,287 8. Current fallows 1,61,759 9. Net area sown 3,55,674 10. Total Geographical area 14,98,778 11. Total cropped area 4,08,000 12. Area sown more than once 36,283 CHITTOOR DISTRICT FARMING SITUATIONS S. No Farming Situation Total No.of Area (HA) Mandals 1. Medium Irrigation (Canal) Red Soils 15,216 14 2. Minor Irrigation (Tanks) Red Soils 42,368 61 3. -
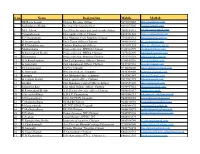
S.No Name Designation Mobile Mail Id
S.no Name Designation Mobile Mail id 1 SK.Razia begum District Revenue Officer 9491077003 [email protected] 2 kodhndarami Reddy Revenue Divisional officer 9491077005 [email protected] 3 M.A. Jaleen Asst.Director.surey and land records.chittor 9866169511 [email protected] 4 Umamaheswar Dist.supply Officer,Chittoor 8008301423 [email protected] 5 E.N.Jayaramulu Dist.Manager,Civil Supplies,Chittoor 7702003533 [email protected] 6 G.Sreenivasulu Divl..Forest Officer,Chittoor 9440810136 [email protected] 7 K.L.Prabhakar rao District Panchayath Officer 9491071325 [email protected] 8 Madhavilatha Project director ,DWMA,Chittoor 9100966779 [email protected] 9 B.Raviprakash Reddy Project director ,DRDA,Chittoor 7675854309 [email protected] 10 Dhananjaya Project director ,Housing,Chittoor 7093930110 [email protected] 11 G.A.Ravichandran Dist.Co-Operative Officer,Chittoor 9100109216 [email protected] 12 K.Samuyelu Dist.Educational Officer,Chittoor 9849909110 [email protected] 13 P.Chandramouli DVEO,Tirupathi 9440816009 [email protected] 14 K.Munnaiah RIO.Intermideate,Tirupathi 9848309000 [email protected] 15 Lavanya Dist.Malariya Officer,chittoor 9849902383 [email protected] 16 G.venkata Prasad Dist.Leprocy officer,Chittoor 9819902375 [email protected] 17 surekha Dist.Blindness control Officer,chittoor 8008553649 [email protected] 18 M.Eswara Rao Dist.tribal welfare officer, Chittoor 9490957021 [email protected] 19 B.Raviprakash Reddy A.D.Disabled welfare officer,Chittoor 9000013617 addwctr@gmail. 20 S.Sreenivaskumar E.D,S.C.Corporation -

Chittoor District 2018
HAND BOOK OF STATISTICS CHITTOOR DISTRICT 2018 ***** Compiled and Published by CHIEF PLANNING OFFICER CHITTOOR DISTRICT Sri PRADYUMNA P.S, I.A.S., District Collector & Magistrate, Chittoor District. PREFACE I am happy to release the Thirty Second Edition of Hand Book of Statistics of Chittoor District which incorporates Statistical Data of various departments for the Year 2018. The Statistical Data in respect of various departments and schemes being implemented in the district are compiled in a systematic and scientific manner reflecting the progress during the year. The sector-wise progress is given in a nutshell under the chapter “DISTRICT AT A GLANCE” apart from Mandal-wise data. The publication reflects the latest data on various aspects of the District Economy. The information has been given Mandal-wise in a concise form to facilitate an overall assessment of the District Economy for the year. The current publication unveils the present scenario of the development in all aspects of different parameters both at District and Mandal Level. This publication is very useful reference book for the General public, Planners, Administrators, Research Scholars, Bankers and also special Agencies who are involved in the formulation and implementation of various developmental programmes in the district. I am thankful to all District Officers and the heads of other institutions for extending their helping hand by furnishing their respective Statistical data to the Chief Planning Officer for publication of this Hand Book. I appreciate the efforts made by Sri. Ch. V.S.Bhaskara Sarma, Chief Planning Officer, Chittoor, other Officers and Staff Members of the Chief Planning Office in bringing out this publication which projects the development of the District during the year 2018. -
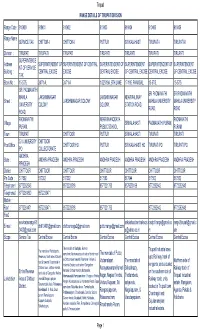
Div and Range Address
Tirupati RANGE DETAILS OF TIRUPATI DIVISION Range Code : 910400 910401 910402 910403 910404 910405 910406 Range Name SERVICE TAX CHITTOR-I CHITTOR-II PUTTUR SRI KALAHASTI TIRUPATI-I TIRUPATI-II : Division : TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI SUPERINTENDE Address SUPERINTENDENT OF SUPERINTENDENT OF CENTRAL SUPERINTENDENT OF SUPERINTENDENT SUPERINTENDENT OF SUPERINTENDENT NT OF SERVICE Building : CENTRAL EXCISE EXCISE CENTRAL EXCISE OF CENTRAL EXCISE CENTRAL EXCISE OF CENTRAL EXCISE TAX Block No : 15-57/5, 24/71-A, 24/71-A 1/325/19A, 6TH LANE 17-195, PANAGAL, 15-57/5, 15-57/5, SRI PADMAVATHI SRI PADMAVATHI SRI PADMAVATHI MAHILA LAKSHMINAGAR LAKSHMI NAGAR NEAR RALIWAY Street : LAKSHMINAGAR COLONY MAHILA UNIVERSITY MAHILA UNIVERSITY UNIVERSITY COLONY, COLONY, STATION ROAD, ROAD, ROAD ROAD, PADMAVATHI NEAR BHANODAYA PADMAVATHI Village : SRIKALAHASTI PADMAVATHI PURAM, PURAM, PUBLIC SCHOOL, PURAM, Town : TIRUPATI CHITTOOR PUTTUR SRIKALAHASTI TIRUPATI TIRUPATI S.V.UNIVERSITY CHITTOOR Post Office : CHITTOOR HO PUTTUR SRI KALAHASTI HO TIRUPATI PO TIRUPATI PO PO COLLECTORATE ANDHRA State : ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH PRADESH District : CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR Pin Code : 517502 517002 517002 517583 517644 517502 517502 Telephone1 : 8772262546 8572220518 8577221753 8578230199 8772262642 8772252643 Telephone2 : 8772261830 8572233471 Mobile : Fax1 : 8772261471 8572233471 8572220518 8577221753 8772262642 8772262643 Fax2 : servicetaxrange91 -

Meos & MIS Co-Ordinators
List of MEOs, MIS Co-orfinators of MRC Centers in AP Sl no District Mandal Name Designation Mobile No Email ID Remarks 1 2 3 4 5 6 7 8 1 Adilabad Adilabad Jayasheela MEO 7382621422 [email protected] 2 Adilabad Adilabad D.Manjula MIS Co-Ordinator 9492609240 [email protected] 3 Adilabad ASIFABAD V.Laxmaiah MEO 9440992903 [email protected] 4 Adilabad ASIFABAD G.Santosh Kumar MIS Co-Ordinator 9866400525 [email protected] [email protected] 5 Adilabad Bazarhathnoor M.Prahlad MEO(FAC) 9440010906 n 6 Adilabad Bazarhathnoor C.Sharath MISCo-Ord 9640283334 7 Adilabad BEJJUR D.SOMIAH MEO FAC 9440036215 [email protected] MIS CO- 8 Adilabad BEJJUR CH.SUMALATHA 9440718097 [email protected] ORDINATOR 9 Adilabad Bellampally D.Sridhar Swamy M.E.O 7386461279 [email protected] 10 Adilabad Bellampally L.Srinivas MIS CO Ordinator 9441426311 [email protected] 11 Adilabad Bhainsa J.Dayanand MEO 7382621360 [email protected] 12 Adilabad Bhainsa Hari Prasad.Agolam MIS Co-ordinator 9703648880 [email protected] 13 Adilabad Bheemini K.Ganga Singh M.E.O 9440038948 [email protected] 14 Adilabad Bheemini P.Sridar M.I.S 9949294049 [email protected] 15 Adilabad Boath A.Bhumareedy M.E.O 9493340234 [email protected] 16 Adilabad Boath M.Prasad MIS CO Ordinator 7382305575 17 Adilabad CHENNUR C.MALLA REDDY MEO 7382621363 [email protected] MIS- 18 Adilabad CHENNUR CH.LAVANYA 9652666194 [email protected] COORDINATOR 19 Adilabad Dahegoan Venkata Swamy MEO 7382621364 [email protected] 20 -

Government of Andhra Pradesh
GOVERNMENT OF ANDHRA PRADESH NOTIFICATION Notification by the Government of Andhra Pradesh Office of the District Collector & District Magistrate, Chittoor District It is hereby declared that the areas detailed in the notification are made available free for Sand to the Public with immediate effect. 1) List of open excavation of Sand reaches : Approximate Quantity in Mandal Name of Reach Cbm the specified area B.Kothakota 8400 Berangi 8620 Bangaruvaripalli 8500 Ghattu 6500 Thummanagutta 7500 B.Kothakota Gollapalli 9850 Kottavooru 7485 Seelamvaripalli 9800 Gummasamudram 8500 Gangapuram 8500 Kaalangi River near Pallamala 7400 B.N.Kandriga Village Alathur 9600 Sanambatla 13000 Chandragiri Sanambatla 7560 Gajuleru Vanka of YV Palem 9100 Chinnagottigallu Kappaleru Vanka of YV Palem 8200 Anagallu - Reach -1 19500 Anagallu - Reach -2 19150 Chittoor Peddavanka 1500 Ananthapuram 9000 Muthukuru 9200 Gargeya River 9500 Chowdepalli A.Kothakota 8800 G.D.Nellore Nandanur 19500 (Contd…2) -2- Koundiny River Vanka near Gangavaram 8600 Nidigunta of Peddapanjani Vinayakavaripalli, Ramapuram Gurramkonda 9600 GP of Gurramkonda Irala Eguvacheruvu 5000 Zillelemanda 155000 K.V.Palli T.Sundupalli of KV Palli 8500 Gangapuram 25970 Kalakada Nallaguttapalli of Balaiahgaripalli 9200 Mahal 21960 Cheekatipalli - Reach 1 19385 Cheekatipalli - Reach 2 19498 Addavaripalli - Reach 1 19330 Addavaripalli - Reach 2 19075 Kalikiri Gundluru - Reach 1 18000 Gundluru - Reach 2 19300 Medikurthi 20090 Parapatla - Reach 1 19300 Parapatla - Reach 2 19500 Chinthaparthy of Valmikipuram -

KURNOOL K Venkateswara
ANDHRA PRADESH POLLUTION CONTROL BOARD ZONAL OFFICE: KURNOOL 1st Floor, Shankar Shopping Complex, Krishna Nagar Main Road Phone :08518- 233619, e-mail: [email protected] CONSENT ORDER FOR ESTABLISHMENT Order No.CTR- 1550/PCB/ZO-KNL/CFE/2019 Dt.19.08.2019 Sub: PCB-ZO-KNL-CFE - Sri. M.S.Manjunath, Sy.No. 450/18 (P), Gorivimakulapalli (V), Ramakuppam (M), Chittoor District, Andhra Pradesh – Consent for Establishment (CFE) Order of the Board under Sec.25 of Water (Prevention & Control of Pollution) Act, 1974 and under Sec.21 of Air (Prevention & Control of Pollution) Act, 1981- Order- Issued- Reg. Ref: 1. CFE application received through OCMMS on 28.07.2019 2. R.O.TPT Lr.No.C-2094/PCB/RO/TPT/2019 -1193, dt: 03.08.2019 3. CFE Committee meeting held at ZO, Kurnool on 17.08.2019. ***** 1) In the reference 1st cited, an application was submitted to the Board seeking Consent for Establishment (CFE) to carryout mining activity of the following mineral with installed capacity as mentioned below, with a total project cost of Rs.55.0 Lakhs Sl.No. Activity Capacity 1. Mining of Black granite 250 m3/annum ( Mine lease area – 1.0 Ha) 2) As per the application, the above mining activity is to be carried out at Sy.No. 450/18 (P), Gorivimakulapalli (V), Ramakuppam (M), Chittoor District, Andhra Pradesh in the mine lease area of 1.0 Ha. 3) The above site was inspected by Environmental Engineer & Asst. Environmental Engineer, APPCB, Regional Office, Tirupati on 01.08.2019 and found that it is surrounded by: North : Dry lands South : Dry lands East : Dry lands West : Dry lands 4) The Board, after careful scrutiny of the application and verification report of Regional Office, hereby issues CONSENT FOR ESTABLISHMENT(CFE) to your mine under section 25 of Water (Prevention & Control of Pollution) Act, 1974 and under Section 21 of Air (Prevention & Control of Pollution) Act, 1981 and the rules made there under. -

Hand Book of Statistics Chittoor District
HAND BOOK OF STATISTICS 1 9 8 7 - 88 CHITTOOR DISTRICT COMPILED AND PUBLISHED BY CHIEF PLANNING OFFICER CHITTOOR I > J 7 Tt H vf"..P i "-dx. U ''v \ i" ■ " ‘ f1 I ? w ^\! ''-I T '- - - - ' # i, '■X A > '-Y'^ > » s ? V i I : I I '? I ? I /.- "r--t--l I ' - - J !> . I'-,,,, SV/ I Q \ . 5.5 I J 1 .1 I t •..••I S./ I ' LY •?-- 0S !.-^ 5 I U " ^ ‘i I |> . ■• I I a •>«s*si < §K' j ^ - K / -•i< s i r " r 5 - ‘' : i / - ’, . ! V s , > 9 d b . N . Nttiou..] ,..y ; j'JlECJtiom|\ ^ ■ D - ^ " 3 € ^ ................ I 3 > 1 ^ J C.VISW ANATH, i a s , Disric: Collector and Magistrate, Chtocr. P r e f a c e The.:Present issue of Hand Bpok of Statistics, Chittococ'^Bisirict for the year 1987^68 is the Sixth in its series of'publications. It contains dat&, on various aspeCts'’df'tfie district'economy. The publication will serve as a useful reference book for the general public, researchers, planners, administrators and bankers. I am thankful to all the district o_^cers, officers of the Revenue Deptirtmmt and 0ea^ ^ Insti fo r their co-^'p^tdilhH in furnistiing the^-data. T h ie ff orts made by the Chief Fiatining Officer and his staff for preparation and. publication of this Hand Book are appreciated. Any suggestions for the improvement of this publication are welcome. ..fiHTTOOR %)-l-1989 Contents Table No. PARTICULARS Pages Historical Background of the Chittoor District i—v Comparison of the District ‘ with the State 1987-88 vi—vii Administrative Divisions in the District as on 31-3-88 viii—ix Members of Parliament and Legislative Assembly in Chittoor District ^ Name of Chairman, Zilla Praja Parishad and Presidents of Mandal Praja Parishad in the District xi—xii 1. -

Territorial Jurisdiction of Civil Courts
TERRITORIAL JURISDICTION OF CIVIL COURTS I. JURISDICTION OF DISTRICT COURT AND ADDITIONAL DISTRICT COURTS AT CHITTOOR. S.No Court Name Name of Mandals 1. Chittoor (Urban and Rural). 2. Bangarupalem. 3. Yadamari. 4. Gudipala. 5. Thavanampalli. i. District and Sessions Court, Chittoor. 6. Puthalapat. 7. G.D.Nellore. ii. I Additional District and Sessions Court, 8. Penumur. Chittoor. 9. Irala. 10. Pakala. 1. iii. VIII Additional District and Sessions Court, 11. Pulicharla. Chittoor. 12. Palamaner. 13. Gangavaram. iv. IX Additional District and Sessions Court, 14. Baireddipalle. Chittoor. 15. V. Kota. 16. Kuppam. 17. Gudipalli. 18. Santhipuram. 19. Ramakuppam II. JURISDICTION OF ADDITIONAL DISTRICT COURTS AT MADANAPALLE. S.No Court Name Name of Mandals 1. Madanapalle Urban and Rural. 2. Nimmanapalle. 3. Kurabalakota . 4. B.Kothakota. i. II Additional District & Sessions Court, 5. Thamballapalle. Madanapalle. 6. Mulakalacheruvu. 2. ii. VII Additional District & Sessions Court, 7. Peddathippa Samudram. Madanapalle. 8. Peddamandyam. 9. Punganur. 10. Ramasamudram. 11. Chowdepalle. 12. Peddapanjani. 13. Somala. III. JURISDICTION OF ADDITIONAL DISTRICT COURTS AT TIRUPATI. S.No Court Name Name of Mandals i. III Additional District & Sessions Court, 1. Tirupati Urban & Rural. Tirupati, Special Court for U/s. 4 of the 2. Chandragiri. Prevention of Money Laundering Act, 2002 3. Renigunta. (Act 15 of 2003) for the Districts of Chittoor, 4. Puttur. Ananthapur, Kadapa, Kurnool and Nellore. 5. Narayanavanam. 6. Vadamalpet. ii. IV Additional District and Sessions Court, 7. Vedurukuppam. Tirupati and Special Court for SC&ST 8. Sri Rangarajapuram. (POA) Act, 1989 for Tirupati Revenue 9. Karvetinagaram. Division. 10. Ramachandrapuram. 11. Palasamudram. 3. 12. Nagari. iii. V Additional District and Sessions Court, 13. -

District Survey Report- Chittoor District
District Survey Report – 2018 DEPARTMENT OF MINES AND GEOLOGY Government of Andhra Pradesh DISTRICT SURVEY REPORT- CHITTOOR DISTRICT Prepared by ANDHRA PRADESH SPACE APPLICATIONS CENTRE (APSAC) ITE&C Department, Govt. of Andhra Pradesh 2018 1 District Survey Report – 2018 ACKNOWLEDGEMENT APSAC wishes to place on record its sincere thanks to Sri B.Sreedhar IAS, Secretary to the Government (Mines) and the Director, Department of Mines and Geology, Govt. of Andhra Pradesh for entrusting the work for preparation of District Survey Reports of Andhra Pradesh. The team gratefully acknowledge the help of the Commissioner, Horticulture Department, Govt. of Andhra Pradesh and the Director, Directorate of Economics and Statistics, Planning Department, Govt. of Andhra Pradesh for providing valuable statistical data and literature. The Project team is also thankful to all Joint Directors, Deputy Directors, Assistant Directors and the staff of Mines and Geology Department for their overall support and guidance during the execution of this work. Also sincere thanks are due to the scientific staff of APSAC who has generated all the thematic maps. VICE CHAIRMAN APSAC 2 District Survey Report – 2018 S Contents Page No 1 Salient Features of Chittoor District 1 1.1 Administrative Setup 1 1.2 Drainage & Physiography 3 1.2a Drainage 3 1.2b Physiography 3 1.3 Climate & Rainfall 5 1.3a Climate 5 1.3b Rainfall 5 1.3c Mean monthly rainfall distribution 6 1.4 Trasport & communication net work of chittoor district 12 1.5 Population and literacy 13 1.6 Important places of Tourism 15 2 2.1 Land Utilisation of Chittoor District 20 2.1a Land Use / Land Cover 20 2.1b 22 Analysis 2.1c Eco sensitive areas 31 2.2 Slope Map of the District 34 2.3 Forest Cover Distribution 37 3 Agriculture and Soil Resources 38 3.1 Kharif Rice Estimation using SAR data 38 3.2 Soils 39 3.2.A Salt-affected land 40 3.3 Horticulture 40 4 Water Resources in the Chittoor District 45 4.1 Surface Water And Irrigation Resorces of The District 45 4.1.a River Basins in Chittoor District 45 4.2.0. -

Doubling Farmers' Incomes Through Grafted Vegetable Seedlings
Annual Progress Report August 2019- July 2020 Doubling Farmers’ Incomes through Grafted Vegetable Seedlings Submitted to the Department of Horticulture Government of Andhra Pradesh Contents Executive summary ................................................................................................................................. 1 Background ............................................................................................................................................. 1 Scope ....................................................................................................................................................... 1 Objectives ............................................................................................................................................... 1 Consortium partners and institutional arrangements ............................................................................ 2 Strategy ................................................................................................................................................... 2 Update on project activities .................................................................................................................... 3 Annexures ............................................................................................................................................. 18 Executive summary The COVID-19 pandemic and the consequent nationwide lockdown brought to a halt livelihood opportunity across all sectors. Even though the