Assessment Report on Salix [Various Species Including S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Reference Guide Contents Page
PRODUCT REFERENCE GUIDE CONTENTS PAGE INTRODUCTION ����������������������������������������������������������������������������������������������������������������������������������������������� 3 PRODUCT INFORMATION ������������������������������������������������������������������������������������������������������������������������������� 4 HOW NATURAL INGREDIENTS WORK ���������������������������������������������������������������������������������������������������������� 5 OUR KEY INGREDIENTS���������������������������������������������������������������������������������������������������������������������������� 6 - 7 PRODUCT SPECIFICATIONS ��������������������������������������������������������������������������������������������������������������������� 8 - 9 MILK CLEANSER �������������������������������������������������������������������������������������������������������������������������������������������� 10 ENZYME GEL CLEANSER ����������������������������������������������������������������������������������������������������������������������������� 11 ENZYME EXFOLIANT POWDER ������������������������������������������������������������������������������������������������������������������� 12 VITAMIN A SERUM ����������������������������������������������������������������������������������������������������������������������������������������� 13 VITAMIN C SERUM ����������������������������������������������������������������������������������������������������������������������������������������� 14 VITAMIN B SERUM -

Intensive Hand Cream
_____________________________________________________________________________________ INTENSIVE HAND CREAM with Alpha Bright Peptide and Environmental Protection • An effective hand treatment to help fade dark spots and provide maximum hydration • Key Ingredients: Green Tea, Coffee Extract, Shea Butter, Alpha Bright Peptide DESCRIPTION Our hands give away our age, influenced by stress, environmental pollutants and long-term sunlight exposure. This hand cream helps to fade dark spots and provide maximum hydration. A powerful blend of Green Tea, Coffee Extract and Alpha Bright Peptide helps to preserve a youthful appearance of your hands by protecting them from free radical and environmental impact. Absorbs easily, non-greasy. FEATURES AND BENEFITS • Alpha Bright Peptide, blended with Camellia Sinensis (Green Tea) Extract, and Salix alba (Willow Bark) Extract for effective skin lightening. • Butyrospermum Parkii (Shea) Butter and Rosa Canina (Rosehip) Fruit Oil for moisturizing and soft hands. • Coffea Robusta (Coffee) Extract and Ascorbic Acid (Vitamin C) restore skin elasticity and allow for a youthful appearance. • Lavandula Angustifolia Flower (Leaf) Stem Oil and Rosmarinus Officinalis (Rosemary) Extract to provide the natural scent of the hand cream. USAGE Apply to clean hands as often as you like. INGREDIENTS: Water (Agua), Stearyl Alcohol, Glyceryl Stearate SE, Alpha Bright Peptide Pal-CR, Ceteareth-20, Glycerine, Propanediol (Zemea), Canola Oil, Silica, Zea Mays (Corn) Starch, Butyrospermum Parkii (Shea) Butter, Sorbitan Stearate, -

Certificate of Analysis
makingcosmetics Certificate of Analysis Product Name: Body Butter Creme Base INCI Name: Or ganic Aloe Leaf Juice (Aloe Barbadensis), Organic Shea Butter (Butyrospermum Parkii), Emulsifying Wax (Cetyl Alcohol, Stearyl Alcohol, Polysorbate 60), Glycerin, Witch Hazel Water (Hamamelis Virginiana), Cocoa Butter (Theobroma Cacao), Stearic Acid, Phenoxyethanol, Vitamin E (Tocopherol), Sunflower Seed Oil (Helianthus Annuus), Rose Flower Water (Rosa Damascena), Mango Seed Butter (Mangifera Indica), Organic White Willow Bark (Salix Alba), Organic Rosemary Leaf Extract (Rosmarinus Officinalis), Organic Sunflower Seed Oil (Helianthus Annuus), Organic Neem Seed Oil (Melia Azadirachta), Sweet Orange Peel Oil (Citrus Sinensis), Lavender Flower Oil (Lavandula Angustifolia), Xanthan Gum, Alcohol, Tetrasodium Glutamate Diacetate. Lot Number: Not avai lable (data may vary slight ly wi th different lots or batches) CAS Number: N/A Shelf Life : 24 months from manufacture date Specifications Range Res ults Appearance Thick, Creamy Pass Color White Pass Odor Citrus, Flora l Pass pH at 25.1 °C 5.17 Pass Viscosity (Brookfield ) 11520 cPs Pass Plate Count Bacteria <10 CFU/mL Pass Yeasts & Molds <10 CF U/mL Pass The above data were obtained using the test indicated and is subject to the deviation inherent in the test method. Results may vary under other test methods or conditions. This report is not to be signed. All data are as per our supplier. Disclaimer : This information relates only to the specific material designated and may not be valid for such material used in combination with any other materials or in any other process. Such information is to be the best of the company’s knowledge and believed accurate and reliable as of the date indicated. -

White Willow (Salix Alba)
CAMPO RESEARCH PTE LTD Level 30, 6 Battery Road, Singapore 049909 Tel: (65) 63833203 / 202 / 63833631 Direct Fax (65) 63833632 / 63834034 Email: [email protected] Website: http///www.campo-research.com CAMPO® Multi-Purpose Cosmetic Base Chemicals & Active Ingredients CAMPO® Novel Functional Active Cosmetic Ingredient & Raw Materials Campo Willow Liposomal Extracts 2 INDEX An Extract of Salix alba; An Efficacious Safe Remedy for Problem Skin Next generation of skin care for aging skin? Materials and Methods Discussion Conclusion SKIN REVEALING LOTION FOR PROBLEM SKIN White Willow (Salix alba) TECHNICAL SPECIFICATIONS (CAMPO LIPOSOMAL WILLOW EXTRACT) MATERIAL SAFETY DATA SHEETS (CAMPO LIPOSOMAL WILLOW EXTRACT) TECHNICAL SPECIFICATIONS (CAMPO WILLOW EXTRACT) MATERIAL SAFETY DATA SHEETS (CAMPO WILLOW EXTRACT) IMPORTANT NOTICE Specifications may change without prior notice. Information contained in this technical literature is believed to be accurate and is offered in good faith for the benefit of the customer. The company, however, cannot assume any liability or risk involved in the use of its natural products or their derivatives, since the conditions of use are beyond our control. Statements concerning the possible use are not intended as recommendations to use our products in the infringement of any patent. We make no warranty of any kind; expressed or implied, other than that the material conforms to the applicable standard specifications. Ask about our Herbal Natural Products Chemistry Consultancy Services – Product Registration EEC/UK New Drug Development (NDA-US); Quasi-Drug Topicals (MOHW_Japan); Development of Standards, Analysis & Profiles of Phytochemicals; Literature searches, Cultivation of Medicinal Plants, Clinical-Trials, Development of new uses for Phytochemicals and Extracts; Contract Research and Development Work in Natural Products for Novel Drugs, New Cosmetic Active Ingredients for Active Topica/OTC Cosmetic with functionality and Consumer-perceivable immediate-results, New Food Ingredients for Nutraceuticals & Functional Foods. -

Terre Mere Ingredients List
Terre Mere Ingredients List Jojoba Tea Clarifying Cleanser - Combination-Oily Skin: Aloe barbadensis (Organic Aloe) Juice, Aspalathus linearis (Organic Rooibos Tea) Extract, Olea europaea (Organic Olive) Oil, Cocamidopropyl Betaine, Emulsifying Wax, Vegetable Glycerin, Methylsulfonylmethane (MSM), Simmondsia chinensis (Organic Jojoba) Oil, Decyl Glucoside, Dimethylaminoethanol (DMAE), Camellia sinensis (Organic Green Tea) Extract, Tocopherol (Vitamin E),Sodium Hyaluronate (Hyaluronic acid), Activated Charcoal, Camellia sinensis (Organic White Tea) Extract, Usnea (Lichen) Extract, Salix alba (Willow Bark) Extract, Panthenol (Vitamin B Complex), Calophyllum inophyllum (Tamanu) Oil, Ascorbyl Palmitate (Vitamin C Ester), Xanthan Gum (Polysaccharide Gum), (May contain sodium bicarbonate and/or citric acid as pH adjusters). Apple Cider Vinegar Toner - Combination-Oily Skin: Aloe barbadensis (Organic Aloe) Leaf Juice, Acetic Acid (Apple Cider Vinegar), Salix alba (Willow Bark) Extract, Phenoxyethanol, Polysorbate, Melaleuca alternifolia (Tea Tree) Beauty Essentials for Essential Oil, Tetrasodium EDTA, Polysorbate, (May contain sodium Combination to Oily Skin 3- bicarbonate and/or citric acid as pH adjusters). Piece Set Green Tea and Chamomile Moisturizer - Combination-Oily Skin: Aloe barbadensis (Organic Aloe) Juice, Lavendula angustifolia (Organic Lavender) Distillate, Anthemis nobilis (Roman Chamomile) Distillate, Cocos nucifera (Organic Coconut) Oil, Emulsifying Wax, Palm Stearic Acid, Vegetable Glycerin, Simmondsia chinensis (Jojoba) Oil, -

Extensive Willow Biomass Production on Marginal Land
Pol. J. Environ. Stud. Vol. 28, No. 6 (2019), 4359-4367 DOI: 10.15244/pjoes/94812 ONLINE PUBLICATION DATE: 2019-07-29 Original Research Extensive Willow Biomass Production on Marginal Land Mariusz J. Stolarski*, Stefan Szczukowski, Józef Tworkowski, Michał Krzyżaniak University of Warmia and Mazury in Olsztyn, Faculty of Environmental Management and Agriculture, Department of Plant Breeding and Seed Production, Olsztyn, Poland Received: 14 June 2018 Accepted: 3 September 2018 Abstract In temperate climate zones, fast-growing willow species harvested in short harvest rotations of 3 to 10 years are an interesting source of biomass for energy or industrial purposes. The aim of this study was to determine morphological traits and biomass yields of three willow cultivars and three clones cultivated on three different types of marginal soils at two densities. Willow was grown in the Eko-Salix system, with no ploughing, with limited fertilization and cultivation measures, harvested in a 7-year rotation. The experiment showed that willow can be produced in the Eko-Salix system in extensive cultivation; however, the yield was strongly differentiated by the marginal soils and by the cultivars and clones under study and ranged from 4.4 to 17.8 Mg ha–1 year–1 DM. The mean yield from all the sites for all the cultivars and clones as well as planting densities in the experiment was 8.0 Mg ha–1 year–1 DM. The biomass yield obtained on peat-muck soil and humic alluvial soil was similar and significantly higher than on very heavy clay soil. The Ekotur cultivar gave plants with better morphological traits and the significantly highest mean yield (12.9 Mg ha–1 year–1 DM). -

CMEC 70 Extracted Ratified Minutes
CMEC 70 COMPLEMENTARY MEDICINES EVALUATION COMMITTEE EXTRACTED RATIFIED MINUTES SEVENTIETH MEETING 12 DECEMBER 2008 ABBREVIATIONS ADI Acceptable Daily Intake ADRAC Adverse Drug Reactions Advisory Committee ADRs Adverse Drug Reactions ADRU Adverse Drug Reaction Unit AHS Australian Approved Herbal Name ARTG Australian Register of Therapeutic Goods ASMI Australian Self-Medication Industry Organisation BP British Pharmacopoeia BWI Bovine Whey Immunoglobulins CAM Complementary and Alternative Medicines CATAG Council of Australian Therapeutics Advisory Group CHC Complementary Healthcare Council CMEC Complementary Medicines Evaluation Committee EAA Excitatory Amino Acid EAG Expert Advisory Group EFSA European Food Safety Authority EP European Pharmacopoeia EU European Union FSANZ Food Standards Australia New Zealand IP Intraperitoneal CMEC 70 Extracted Ratified Minutes IU International Units IV Intravenous JECFA Joint FAO/WHO Expert Committee on Food Additives LFT Liver Function Test LOEL Lowest Observed Effect Level MEC Medicine Evaluation Committee NDPSC National Drugs and Poisons Schedule Committee NH&MRC National Health and Medical Research Council NOEL No Observed Effect Level NPNZ Natural Products New Zealand OCM Office of Complementary Medicines OICG Office of Complementary Medicines/ Industry Consultation Group OMSM Office of Medicines Safety Monitoring PPRC Pharmacopoeia of the People’s Republic of China RDI Recommended Daily Intake SATCM State Drug Administration of the Republic of China SDS-PAGE Sodium dodecyl sulphate – polyacrylamide gel SUSDP Standard for the Uniform Scheduling of Drugs and Poisons TCM Traditional Chinese Medicine TGA Therapeutic Goods Administration TGO Therapeutic Goods Order UWS University of Western Sydney The Complementary Medicines Evaluation Committee (CMEC) held its seventieth meeting in the Hilton Hotel, Melbourne Airport, from 9.30 a.m. to 4 p.m. -

|||||||||||III USOO565932A United States Patent (19) 11 Patent Number: 5,165,932 Horvath 45 Date of Patent: Nov
|||||||||||III USOO565932A United States Patent (19) 11 Patent Number: 5,165,932 Horvath 45 Date of Patent: Nov. 24, 1992 (54) THERAPEUTICAL COMPOSITIONS 56) References Cited AGAINST PSORASIS U.S. PATENT DOCUMENTS 4,569,839 2/1986 Grollier et al. .................. 424/195.1 75) Inventor: E. Horvath, Szalai Attila, 4,758,433 7/1988 Johnson et al. .................. 424/195.1 ungary OTHER PUBLICATIONS 73) Assignee: Unipharma Co., Ltd., Budapest, “The Herb Book'; John B. Lust, First Ed. Bendedict Hungary Lust Publications. Primary Examiner-John W. Rollins 21 Appl. No.: 397,429 Attorney, Agent, or Firm-Ladas & Parry 57 ABSTRACT 22 PCT Filed: Dec. 23, 1987 The invention relates to therapeutical compositions on medical herb basis for the treatment of psoriasis and the 86 PCT No.: PCT/HU87/00060 preparation of the same. A further object of the inven S371 Date: Sep. 15, 1989 tion in the use of medical herbs as listed below in the treatment of psoriasis, rheumatism and asthmatic dysp S 102(e) Date: Sep. 15, 1989 noea. The medical herbs used in the invention are as follows: 87 PCT Pub. No.: WO89/05651 Allium sativum. /garlic/, Urtica dioica /common nettle/, PCT Pub. Date: Jun. 29, 1989 Chelidonium majus /milkweed/, Veronica officinalis /veronica/, 51 int. Cl. .............................................. A61K 35/78 Calendula officinalis /calendula or marigold/, 52 U.S. Cl. ................................. 424/195.1; 514/863; Achillea herba /millefolium/ /yarrow/, 514/886; 514/887 Fumaria officinalis /fumitory, earth-gall/. 58 Field of Search ..................... 424/195.1; 514/886, 514/887, 863 1 Claim, No Drawings 5,165,932 1 2 of different medicinal herbs. -

Botanical Survey of Medicinal Plants Used in the Traditional Treatment of Human Disease in Montain Hay Meadows from Gurghiului Mountains
ABMJ 2019, 2(1): 38-46 DOI: 10.2478/abmj-2019-0005 Acta Biologica Marisiensis BOTANICAL SURVEY OF MEDICINAL PLANTS USED IN THE TRADITIONAL TREATMENT OF HUMAN DISEASE IN MONTAIN HAY MEADOWS FROM GURGHIULUI MOUNTAINS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2, Sanda COŞARCĂ1, Mariana HIRIŢIU1, Florentina OROIAN3, Corneliu TANASE1 1Department of Fundamental Pharmaceutical Sciences, Discipline of Pharmaceutical Botany, University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureș, Romania 2Mureş County Museum, Department of Natural Sciences, Târgu Mureş, Romania 3The Pharmacy Remedia Târgu Mureş, Romania *Correspondence: Silvia OROIAN [email protected] Received: 14 May 2019; Accepted: 15 June 2010; Published: 30 June 2019 Abstract: The aim of this study was to identify the medicinal and aromatic plants from mountain hay meadows (6520 - Natura 2000 habitat) of Gurghiului Mountains and to analyze the correlation of these herbs with their therapeutic compounds as well as the human diseases on which they can be used on therapeutic purpose. The area covered by this study was the Gurghiului Mountains. Regarding the vegetation, this area is characterized by the predominance of forest ecosystems, along with semi-natural mountainous grasslands. The floristic inventory for the studied area included numerous medicinal plants with therapeutic chemical compounds. These medicinal plants were grouped in this study according to the dominant active principles used in phytotherapy. Two plant associations were identified: Festuco rubrae-Agrostietum capillaris Horvat 1951 and Poo-Trisetetum flavescentis Knapp ex Oberdorfer 1957. This survey demonstrates that the medicinal plant area in the Gurghiului Mountains is a promising economic resource for developing this region, but it needs planned exploitation. Keywords: grasslands, habitats, medicinal plants. -

Salix Alba L
Forest Ecology and Forest Management Group Tree factsheet images at pages 3, 4, 5 Salix alba L. taxonomy author, year Linnaeus 1753 synonym Family Salicaceae Eng. Name White willow Dutch name Schietwilg subspecies varieties hybrids Salix x rubens Schrank (S. alba x S. fragilis) cultivars, frequently used ‘Barlo’ timber tree, landscape tree, street tree, park tree ‘Belders’ timber tree, landscape tree, street tree, park tree ‘Bredevoort’ timber tree, landscape tree, street tree, park tree ‘Chermesina’ street tree, park tree ‘Het Goor’ timber tree, landscape tree, street tree, park tree ‘Lievelde’ timber tree, landscape tree, street tree, park tree ‘Tinaarlo’ timber tree, landscape tree, street tree, park tree references Weeda, E.J. 2003. Nederlandse Oecologische Flora, deel 1 Hiemstra, J.A. 2002. Rassenlijst bomen Pas, J.B. van der. 1989. Salix. in: P. Schmidt. 1989. Nederlandse boomsoorten 2 Plants for a Future Database; www.pfaf.org/index.html morphology crown habit tree, oval max. height (m) 30 max. dbh (cm) >100 actual size Great Britain year …, d(…) 238, h 10, Amberley Wild Brook, West Sussex, England year …, d(…) 235, h 23, Moreton-on-Lugg, Herefordshire, England actual size Netherlands year 1830-1840, d(130) 115, h 23 year 1920-1930, d(130) 162, h 30 year 1930-1940, d(130) 204, h 24 leaf length (cm) 4-13 leaf petiole (cm) 0,2-1 leaf colour upper surface green leaf colour under surface grayish- or blueish green leaves arrangement alternate flowering April flowering plant dioecious flower monosexual flower diameter (cm) flower male catkins length (cm) 3,7-5,5 flower female catkins length (cm) 3,7-5,5 pollination insects fruit; length capsule (doosvrucht); 0,2-0,3 cm fruit petiole (cm) <0,1 seed; length seed; . -
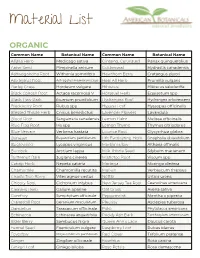
Material List
Material List ORGANIC Common Name Botanical Name Common Name Botanical Name Alfalfa Herb Medicago sativa Ginseng, Cultivated Panax quinquefolius Anise Seed Pimpinella anisum Goldenseal Hydrastis canadensis Ashwagandha Root Withania somnifera Hawthorn Berry Crataegus piperi Astragalus Root Astragalus membranaceus Heal All Herb Prunella vulgaris Barley Grass Hordeum vulgare Hibiscus Hibiscus sabdariffa Black Cohosh Root Actaea racemosa V Horsetail Herb Equisetum spp Black Haw Bark iburnum prunifolium Hydrangea Root Hydrangea arborescens Blackberry Root Rubus spp Hyssop Leaf Hyssopus officinalis Blessed Thistle Herb Cnicus benedictus Lavender Flowers Lavandula Blood Root Sanguinaria canadensis Lemon Balm Melissa officinalis Blue Flag Root Iris spp Lemon Thyme Thymus citriodorus Blue Vervain Verbena hastata Licorice Root Glycyrrhiza glabra Boneset Eupatorium perfoliatum Life Everlasting Herb Gnaphaliu obiusifolium Bugleweed Lycopus virginicus Marshmallow Althaea offinalis Burdock Arctium lappa Milk Thistle Seed Silybum marianum Butternut Bark Juglans cinerea Mistletoe Root Viscum spp Catnip Herb Nepeta cataria Moringa Moringa oleifera Chamomile Chamomilla recutita Mullein Verbascum thapsus Chaste Tree Berry Vitex agnus-castus Nettle Urtica urens Chicory Root Cichorium intybus New Jersey Tea Root Ceanothus americana Cleavers Herb Galium aparine Oat Grass Avena sativa Comfrey Symphytum officinale Peppermint Mentha x piperita Cranesbill Root Geranium maculatum Pleurisy Asclepias tuberosa Dandelion Taraxacum officinale Poke Phytolacca americana -

The Willow Scab Fungus, Fusicladuim Saliciperdum
BULLETIN 302 MARCH, 1929 THE WILLOW SCAB FUNGUS Fusicladium saliciperdum THE WILLOW SCAB FUNGUS Fusicladium saliciperdum G. P. CLINTON AND FLORENCEA. MCCORMICK The Bulletins cf this Station are mailed free to citizens of Connecticut who apply for them, and to other applicants as far as the editions permit. CONNECTICUT AGRICULTURAL EXPERIMENT STATION OFFICERS AND STAFF BOARD OF CONTROL His Excellency, Governor John H. Trumbull, ex-oficio President George A. Hopson, Secretary. ............................Mt. Carmel Wm. L. Slate, Director and Treasurer. ................... .New Haven osephW.Alsop .............................................Avon klijah Rogers.. .......................................Southington Edward C. Schneider. ..................................Middletown Francis F. Lincoln. .......................................Cheshire STAFF. E. H. JENKINS,PH.D., Direclor Emerilus. Administration. WM. L. SLATE,B.Sc., DCeclor and Treasurer. MISS L. M. BRAUTLECHT,Bookkeeper and Librarian. G. E. GRAHAM,In charge of Buildings and Grounds. Chemistry: E. M. BAILEY,PH.D., Chemist in Charge. Analytical C. E. SHEPARD Labcratory. OWENL. NOLAN HARRYJ. FISHER,A.B. Assklanl Chemists. W. T. MATHIS 1 DAVID C-WA~DEN,B.S. j FRANKC. SHELDONLaboratory Assislanl. V. L. CHURCHILI.,&ling Agenl. MRS. A. B. VOSBURGH,Secretary. Biochemical H. B. VICKERY,PH.D., Biochemist in Charge. Laboratory. GEORGEW. PUCHERPH.D Research Assistanl. MISS HELENC. CA~NON,S.S.,Dielilion. Botany. G P CI.~TONSc.D Bolanist in Charge. E: M. STODDADB S" Pomoiogist MISS FLORENCE'A. 'M;~coRMIcK,~H.D., Palhologisr. HAROLDB. BENDER.B.S., Gradwale Assistant. A. D MCDONNELLGeneial Assislanl. MRS.' W. mr. KELSLY,Secrelary. Entomology. 'A'. E. BRITTONPH.D Enlomologisl in Charge: Stale Enlomologisl. B. H. WALDEI;,B.A~R. M. P. ZAPPE,B.S. Assislanl Enlomologisls. PHILIPGARMAN, PH.D. ROGERB.