Durham Research Online
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lithofacies Palaeogeography of the Late Permian Wujiaping Age in the Middle and Upper Yangtze Region, China
Journal of Palaeogeography 2014, 3(4): 384-409 DOI: 10.3724/SP.J.1261.2014.00063 Lithofacies palaeogeography and sedimentology Lithofacies palaeogeography of the Late Permian Wujiaping Age in the Middle and Upper Yangtze Region, China Jin-Xiong Luo*, You-Bin He, Rui Wang School of Geosciences, Yangtze University, Wuhan 430100, China Abstract The lithofacies palaeogeography of the Late Permian Wujiaping Age in Middle and Upper Yangtze Region was studied based on petrography and the “single factor analysis and multifactor comprehensive mapping” method. The Upper Permian Wujiaping Stage in the Middle and Upper Yangtze Region is mainly composed of carbonate rocks and clastic rocks, with lesser amounts of siliceous rocks, pyroclastic rocks, volcanic rocks and coal. The rocks can be divided into three types, including clastic rock, clastic rock-limestone and lime- stone-siliceous rock, and four fundamental ecological types and four fossil assemblages are recognized in the Wujiaping Stage. Based on a petrological and palaeoecological study, six single factors were selected, namely, thickness (m), content (%) of marine rocks, content (%) of shallow water carbonate rocks, content (%) of biograins with limemud, content (%) of thin- bedded siliceous rocks and content (%) of deep water sedimentary rocks. Six single factors maps of the Wujiaping Stage and one lithofacies palaeogeography map of the Wujiaping Age were composed. Palaeogeographic units from west to east include an eroded area, an alluvial plain, a clastic rock platform, a carbonate rock platform where biocrowds developed, a slope and a basin. In addition, a clastic rock platform exists in the southeast of the study area. Hydro- carbon source rock and reservoir conditions were preliminarily analyzed based on lithofacies palaeogeography. -

References Geological Society, London, Memoirs
Geological Society, London, Memoirs References Geological Society, London, Memoirs 2002; v. 25; p. 297-319 doi:10.1144/GSL.MEM.2002.025.01.23 Email alerting click here to receive free email alerts when new articles cite this article service Permission click here to seek permission to re-use all or part of this article request Subscribe click here to subscribe to Geological Society, London, Memoirs or the Lyell Collection Notes Downloaded by on 3 November 2010 © 2002 Geological Society of London References ABBATE, E., BORTOLOTTI, V. & PASSERINI, P. 1970. Olistostromes and olis- ARCHER, J. B, 1980. Patrick Ganly: geologist. Irish Naturalists' Journal, 20, toliths. Sedimentary Geology, 4, 521-557. 142-148. ADAMS, J. 1995. Mines of the Lake District Fells. Dalesman, Skipton (lst ARTER. G. & FAGIN, S. W. 1993. The Fieetwood Dyke and the Tynwald edn, 1988). fault zone, Block 113/27, East Irish Sea Basin. In: PARKER, J. R. (ed.), AGASSIZ, L. 1840. Etudes sur les Glaciers. Jent & Gassmann, Neuch~tel. Petroleum Geology of Northwest Europe: Proceedings of the 4th Con- AGASSIZ, L. 1840-1841. On glaciers, and the evidence of their once having ference held at the Barbican Centre, London 29 March-1 April 1992. existed in Scotland, Ireland and England. Proceedings of the Geo- Geological Society, London, 2, 835--843. logical Society, 3(2), 327-332. ARTHURTON, R. S. & WADGE A. J. 1981. Geology of the Country Around AKHURST, M. C., BARNES, R. P., CHADWICK, R. A., MILLWARD, D., Penrith: Memoir for 1:50 000 Geological Sheet 24. Institute of Geo- NORTON, M. G., MADDOCK, R. -

Loan Agreement
OFFICIAL DOCUMENTS Public Disclosure Authorized LOAN NUMBER 8927-CN Loan Agreement Public Disclosure Authorized (Guizhou Aged Care System Development Program) between PEOPLE'S REPUBLIC OF CHINA and Public Disclosure Authorized INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT Public Disclosure Authorized LOAN AGREEMENT AGREEMENT dated as of the Signature Date between PEOPLE'S REPUBLIC OF CHINA ("Borrower") and INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT ("Bank"). The Borrower and the Bank hereby agree as follows: ARTICLE I - GENERAL CONDITIONS; DEFINITIONS 1.01. The General Conditions (as defined in the Appendix to this Agreement) apply to and form part of this Agreement. 1.02. Unless the context requires otherwise, the capitalized terms used in this Agreement have the meanings ascribed to them in the General Conditions or in the Appendix to this Agreement. ARTICLE II- LOAN 2.01. The Bank agrees to lend to the Borrower the amount of three hundred five million seven hundred thousand Euro (E305,700,000), as such amount may be converted from time to time through a Currency Conversion ("Loan"), to assist in financing the program described in Schedule 1 to this Agreement ("Program"). 2.02. The Borrower may withdraw the proceeds of the Loan in accordance with Section IV of Schedule 2 to this Agreement. All withdrawals from the Loan Account shall be deposited by the Bank into an account specified by the Borrower and acceptable to the Bank. 2.03. The Front-end Fee is one quarter of one percent (0.25%) of the Loan amount. 2.04. The Commitment Charge is one quarter of one percent (0.25%) per annum on the Unwithdrawn Loan Balance. -

High Quality Development Path of Chinese Sauce-Flavor Baijiu Industry—Analysis Based on Consumption Data
E3S Web of Conferences 251, 01099 (2021) https://doi.org/10.1051/e3sconf/202125101099 TEES 2021 High quality development path of Chinese sauce-flavor Baijiu industry—Analysis based on consumption data Cong Peng1,*, and Xu Guo1 1 School of Economics and Finance, Guizhou University of Commerce, Guiyang, Guizhou, China, 550014 Abstract:As an important part of Baijiu industry in China, Chinese sauce-flavor Baijiu is sought after by the consumer market for its unique taste. However, in recent years, there are still many challenges in the process of industrial development. Based on the analysis of three characteristics of Chinese sauce- flavor Baijiu market and the internal and external environments of industrial development, the internal and external factors of high quality and sustainable development of Chinese sauce-flavor Baijiu industry were discussed from three aspects - concentrated development, green development and diversified development. profits and taxes, the sauce-flavor Baijiu industry has made steady development[1]. In 2019, with the 1 Introduction production of 400,000 kilo-litre (about 5.1% of total Baijiu brewing in China has a long time history dated China’s production), the such type of enterprises back to ancient times. In the course of evolution, kinds accomplished the production value of 134.1 billion yuan, of brewing technology have been created and a unique 2% of the total China’s Baijiu industry value, among liquor culture was formed. The custom of raising a cup which the sauce-flavor Baijiu enterprises of Guizhou of liquor has rooted in Chinese culture for hundreds of province produced 80% of the total China’s amount, years. -

Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea
Biodiversity Data Journal 4: e8013 doi: 10.3897/BDJ.4.e8013 Taxonomic Paper Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea Natalie Dale-Skey‡, Richard R. Askew§‡, John S. Noyes , Laurence Livermore‡, Gavin R. Broad | ‡ The Natural History Museum, London, United Kingdom § private address, France, France | The Natural History Museum, London, London, United Kingdom Corresponding author: Gavin R. Broad ([email protected]) Academic editor: Pavel Stoev Received: 02 Feb 2016 | Accepted: 05 May 2016 | Published: 06 Jun 2016 Citation: Dale-Skey N, Askew R, Noyes J, Livermore L, Broad G (2016) Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea. Biodiversity Data Journal 4: e8013. doi: 10.3897/ BDJ.4.e8013 Abstract Background A revised checklist of the British and Irish Chalcidoidea and Mymarommatoidea substantially updates the previous comprehensive checklist, dating from 1978. Country level data (i.e. occurrence in England, Scotland, Wales, Ireland and the Isle of Man) is reported where known. New information A total of 1754 British and Irish Chalcidoidea species represents a 22% increase on the number of British species known in 1978. Keywords Chalcidoidea, Mymarommatoidea, fauna. © Dale-Skey N et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 2 Dale-Skey N et al. Introduction This paper continues the series of checklists of the Hymenoptera of Britain and Ireland, starting with Broad and Livermore (2014a), Broad and Livermore (2014b) and Liston et al. -

Spatial Correlation Between Type of Mountain Area and Land Use Degree in Guizhou Province, China
sustainability Article Spatial Correlation between Type of Mountain Area and Land Use Degree in Guizhou Province, China Yuluan Zhao 1,2 and Xiubin Li 2,* 1 School of Geographic and Environmental Sciences, Guizhou Normal University, Guiyang 550001, China; [email protected] 2 Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China * Correspondence: [email protected]; Tel.: +86-10-6488-9297 Academic Editors: Fausto Cavallaro and Marc A. Rosen Received: 17 May 2016; Accepted: 24 August 2016; Published: 29 August 2016 Abstract: A scientific definition of the type of mountain area and an exploration of the spatial correlation between different types of mountain areas and regional land use at the county level are important for reasonable land resource utilization and regional sustainable development. Here, a geographic information system was used to analyze digital elevation model data and to define the extent of mountainous land and types of mountain areas in Guizhou province. Exploratory spatial data analysis was used to study the spatial coupling relation between the type of mountain area and land use degree in Guizhou province at the county level. The results were as follows: (1) Guizhou province has a high proportion of mountainous land, with a ratio of mountainous land to non-mountainous land of 88:11. The county-level administrative units in Guizhou province were exclusively mountainous, consisting of eight semi mountainous counties, nine quasi mountainous counties, 35 apparently mountainous counties, 13 type I completely mountainous counties, and 23 type II completely mountainous counties; (2) The land use degree at the county level in Guizhou province have remarkable spatial differentiation characteristics. -

79397-89388 Payment for Watershed Services.Pdf
United Nations Development Programme Country: China PROJECT DOCUMENT Payment for Watershed Services in the Chishui River Basin for the Project Title: Conservation of Globally Significant Biodiversity UNDAF Outcome 1: Government and other stakeholders ensure environmental sustainability, Outcome(s): address climate change, and promote a green, low carbon economy Expected CP Outcome(s): Outcome 4: Low carbon and other environmentally sustainable strategies and technologies are adapted widely to meet China’s commitments and compliance with Multilateral Environmental Agreements; and Outcome 5. The vulnerability of poor communities and ecosystems to climate change is reduced Expected CPAP Output (s): Output 4.1 Policy and capacity barriers for the sustained and widespread adoption of low carbon and other environmentally sustainable strategies and technologies removed, and Output 5.1 A strengthened policy, legal, institutional framework for the sustainable use of land, water, the conservation of biodiversity, and other natural resources in fragile ecosystems is enforced. Executing Entity/Implementing Partner: Ministry of Environmental Protection Implementing Ent ity/Responsible Partners: Environmental Protection Department of Guizhou Brief Description The Chishui River is one of the most important tributaries of the upper Yangtze River, because of its diverse landscapes, richness in biodiversity and abundance in water resources. It is the only major tributary of the Upper Yangtze that remains free-flowing without a mainstream dam. The Chishui River Basin (CRB) is an important storehouse of biodiversity, lying within the Upper Yangtze Freshwater Ecoregion and the Guizhou Plateau Broadleaf and Mixed Forests Terrestrial Ecoregion. The basin also lies on the eastern margin of the Mountains of Southwest China biodiversity hotspot, and contains part of the China Danxia World Heritage Site. -

Bibliographia Trichopterorum
Entry numbers checked/adjusted: 23/10/12 Bibliographia Trichopterorum Volume 4 1991-2000 (Preliminary) ©Andrew P.Nimmo 106-29 Ave NW, EDMONTON, Alberta, Canada T6J 4H6 e-mail: [email protected] [As at 25/3/14] 2 LITERATURE CITATIONS [*indicates that I have a copy of the paper in question] 0001 Anon. 1993. Studies on the structure and function of river ecosystems of the Far East, 2. Rep. on work supported by Japan Soc. Promot. Sci. 1992. 82 pp. TN. 0002 * . 1994. Gunter Brückerman. 19.12.1960 12.2.1994. Braueria 21:7. [Photo only]. 0003 . 1994. New kind of fly discovered in Man.[itoba]. Eco Briefs, Edmonton Journal. Sept. 4. 0004 . 1997. Caddis biodiversity. Weta 20:40-41. ZRan 134-03000625 & 00002404. 0005 . 1997. Rote Liste gefahrdeter Tiere und Pflanzen des Burgenlandes. BFB-Ber. 87: 1-33. ZRan 135-02001470. 0006 1998. Floods have their benefits. Current Sci., Weekly Reader Corp. 84(1):12. 0007 . 1999. Short reports. Taxa new to Finland, new provincial records and deletions from the fauna of Finland. Ent. Fenn. 10:1-5. ZRan 136-02000496. 0008 . 2000. Entomology report. Sandnats 22(3):10-12, 20. ZRan 137-09000211. 0009 . 2000. Short reports. Ent. Fenn. 11:1-4. ZRan 136-03000823. 0010 * . 2000. Nattsländor - Trichoptera. pp 285-296. In: Rödlistade arter i Sverige 2000. The 2000 Red List of Swedish species. ed. U.Gärdenfors. ArtDatabanken, SLU, Uppsala. ISBN 91 88506 23 1 0011 Aagaard, K., J.O.Solem, T.Nost, & O.Hanssen. 1997. The macrobenthos of the pristine stre- am, Skiftesaa, Haeylandet, Norway. Hydrobiologia 348:81-94. -
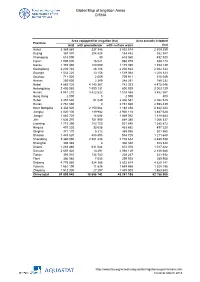
Global Map of Irrigation Areas CHINA
Global Map of Irrigation Areas CHINA Area equipped for irrigation (ha) Area actually irrigated Province total with groundwater with surface water (ha) Anhui 3 369 860 337 346 3 032 514 2 309 259 Beijing 367 870 204 428 163 442 352 387 Chongqing 618 090 30 618 060 432 520 Fujian 1 005 000 16 021 988 979 938 174 Gansu 1 355 480 180 090 1 175 390 1 153 139 Guangdong 2 230 740 28 106 2 202 634 2 042 344 Guangxi 1 532 220 13 156 1 519 064 1 208 323 Guizhou 711 920 2 009 709 911 515 049 Hainan 250 600 2 349 248 251 189 232 Hebei 4 885 720 4 143 367 742 353 4 475 046 Heilongjiang 2 400 060 1 599 131 800 929 2 003 129 Henan 4 941 210 3 422 622 1 518 588 3 862 567 Hong Kong 2 000 0 2 000 800 Hubei 2 457 630 51 049 2 406 581 2 082 525 Hunan 2 761 660 0 2 761 660 2 598 439 Inner Mongolia 3 332 520 2 150 064 1 182 456 2 842 223 Jiangsu 4 020 100 119 982 3 900 118 3 487 628 Jiangxi 1 883 720 14 688 1 869 032 1 818 684 Jilin 1 636 370 751 990 884 380 1 066 337 Liaoning 1 715 390 783 750 931 640 1 385 872 Ningxia 497 220 33 538 463 682 497 220 Qinghai 371 170 5 212 365 958 301 560 Shaanxi 1 443 620 488 895 954 725 1 211 648 Shandong 5 360 090 2 581 448 2 778 642 4 485 538 Shanghai 308 340 0 308 340 308 340 Shanxi 1 283 460 611 084 672 376 1 017 422 Sichuan 2 607 420 13 291 2 594 129 2 140 680 Tianjin 393 010 134 743 258 267 321 932 Tibet 306 980 7 055 299 925 289 908 Xinjiang 4 776 980 924 366 3 852 614 4 629 141 Yunnan 1 561 190 11 635 1 549 555 1 328 186 Zhejiang 1 512 300 27 297 1 485 003 1 463 653 China total 61 899 940 18 658 742 43 241 198 52 -

Back Matter (PDF)
PROCEEDINGS OF THE YORKSHIRE GEOLOGICAL SOCIETY 357 INDEX TO VOLUME 50 General Index density log data, north-west England 93-94 Boreholes depth of burial studies, north-west England 93-99 Chronostratigraphy diagenesis, Permo-Triassic, Cumbria 77-89 Lithostratigraphy dolomitization, Permian, north-east England 151 Localities 'Dumb Fault', north Derbyshire 317-331 Minerals dyke suites, Tertiary, Staffordshire and Shropshire 191-208 New taxa East Irish Sea Basin 74,75,175,178,179 East Irish Sea, post-Triassic structural evolution 93, 98, 99-101 GENERAL INDEX echinoid, Upper Cretaceous, North Yorkshire 115 electron microscopy, Silurian metabentonite, northern Acadian 255-265 England 259-261 aeolian sedimentation, Sherwood Sandstone Group 68-71 faulting, Vale of York 125-128 aeromagnetic modelling, west Cumbria 103-112 Fleswick cycle 178,180-181 Annual Report 1993 185-186 Foredale metabentonite, Acadian cleavage age 257-263 Annual Report 1994 267-268 geochemistry, Cumbria 33-34 apatite fission-track palaeotemperatures, north-west England geochemistry, Permian, north-east England 249-251,252 95-99 geochemistry, Tertiary dyke suites, Staffordshire and Bakevellia Basin, Cumbria 176,177,178,179-181,182 Shropshire 199-203 belemnites, Upper Cretaceous, North Yorkshire 115-118 geophysical log signatures, Permo-Triassic, Cumbria 173-184 biostratigraphy, Cretaceous, North Yorkshire 285-304 glacier, Loch Lomond Stadial, northern Pennines 277-285 biostratigraphy, Dinantian, Cumbria 41,42-43,44 gravity modelling, west Cumbria 103-112 biostratigraphy, Late Triassic, Early Jurassic, Carlisle Basin ignimbrite sequence, caldera-related, west Cumbria 25-36 306-310,314 K-Ar analysis, Silurian metabentonite, northern England biostratigraphy, Namurian, central and northern England 255-256,261-263 333, 335-354 KIRBY, J. -

Transactions of the Shropshire Archaeological and Historical Society
ISSN 0143-5175 Shropshire History and Archaeology Transactions of the Shropshire Archaeological and Historical Society (incorporating the Shropshire Parish Register Society) VOLUME LXXXVII edited by D. T. W. Price SHREWSBURY 2012 (ISSUED IN 2014) © Shropshire Archaeological and Historical Society 2014 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of the Shropshire Archaeological and Historical Society. Produced and printed by 4word Ltd., Bristol COUNCIL AND OFFICERS 1 APRIL 2014 President SIR NEIL COSSONS, O.B.E., M.A., F.S.A. Vice-Presidents ERNIE JENKS MADGE MORAN, F.S.A. M. UNA REES, B.A., PH.D. B. S. TRINDER, M.A., PH.D., F.S.A. Elected Members NIGEL BAKER, B.A., PH.D., F.S.A., M.I.F.A. MARY F. MCKENZIE, M.A., M.AR.AD. NEIL CLARKE, B.A. MARTIN SPEIGHT, B.A., PH.D. ROBERT CROMARTY, B.A. ROGER WHITE, B.A., PH.D., M.I.F.A. HUGH HANNAFORD, M.I.F.A. ANDYWIGLEY, B.SC., M.A., PH.D., F.S.A., P.C.H.E. W. F. HODGES Chairman JAMES LawsON, M.A., Westcott Farm, Habberley, Shrewsbury SY5 0SQ Hon. Secretary and Hon. Publications Secretary G. C. BAUGH, M.A., F.S.A., Glebe House, Vicarage Road, Shrewsbury SY3 9EZ Hon. Treasurer FRANCESCA BUMPUS, M.A., PH.D., 9 Alexandra Avenue, Meole Brace, Shrewsbury SY3 9HT Hon. Membership Secretary PENNY WARD, M.A., M.I.F.A., 1 Crewe Street, Shrewsbury SY3 9QF Hon. -

Annex 1: References
4) NATURAL ENGLAND. 2015. Monitor of Engagement with the Annex 1: References Natural Environment: a pilot for an indicator of visits to the natural Annex 1 environment by children - interim findings from Year 1 (March 2013 to February 2014) (NECR166). http://publications.naturalengland. org.uk/publication/4781405798137856 Section 2: Monitoring the Natural World The Predatory Bird Monitoring Scheme We love the great outdoors and our survey shows it: Monitor of engagement 5) WALKER, L.A., J.S. CHAPLOW, POTTER, E.D., & SHORE, R.F. (2014). with the natural environment PBMS archive holdings: a Predatory Bird Monitoring Scheme (PBMS) report. Lancaster: Centre for Ecology & Hydrology 1) NATURAL ENGLAND, DEFRA & FORESTRY COMMISSION. 2015. Monitor of Engagement with the Natural Environment: The 6) SHORE, R.F., PEREIRA, M.G., POTTER, E.D. and WALKER,. L.A. 2015. national survey on people and the natural environment. https:// Monitoring rodenticide residues in wildlife. In A.P. Buckle and www.gov.uk/government/uploads/system/uploads/ R.H. Smith, eds. Rodent pests and their Control: 2nd edition, 346- attachment_data/file/481299/mene-headline-report-2014-15.pdf 365. Wallingford:CAB International 2) NATURAL ENGLAND. 2015. Visits to the natural environment in East 7) WALKER, L.A., CHAPLOW , J.S., MOECKEL, C., PEREIRA, M.G., London: Analysis of data from the Monitor of Engagement with the POTTER, E.D. & SHORE, R.F. 2015. Anticoagulant rodenticides in Natural Environment survey (2009-13) (NECR 167). sparrowhawks: a Predatory Bird Monitoring Scheme (PBMS) http:// publications.naturalengland.org.uk/ report. Lancaster: Centre for Ecology & Hydrology search?q=NECR167&num=100 8) SHORE, R.F., HENRYS, P.A.