Cross Promotion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Himalayan Kingdoms: Nepal & Bhutan
Exclusive Duke departure – October 30-November 13, 2018 HIMALAYAN KINGDOMS: NEPAL & BHUTAN 15 days from $5,872 total price from Boston, New York, Wash, DC ($5,195 air & land inclusive plus $677 airline taxes and fees) n this exhilarating journey, we discover Otwo distant and devout lands, different in fact yet similar in spirit. Hinduism in Nepal and Buddhism in Bhutan suffuse all aspects of life; in both, tradition and belief abide – and surrounding Himalayan scenery casts a truly awe-inspiring spell. NEPAL Punakha Nagarkot Kathmandu Paro Thimphu BHUTAN Map Legend Destination Air Motorcoach Entry/Departure Avg. High (°F) Oct Nov Kathmandu 80 74 The devout in the Himalayas hang prayer flags to promote peace and compassion. Thimphu 71 64 Paro 66 57 Day 1: Depart U.S. for Kathmandu, Nepal nunnery welcome seekers and visitors to study, medi- tate, and learn about the Mahayana Buddhism practiced Your Small Group Tour Highlights Day 2: Arrive Kathmandu We reach the Nepalese here. After our visit we enjoy a small group highlight capital tonight and transfer to our hotel. as we share lunch with a local family in their home. Opportunity to visit two Himalayan countries • Kathmandu Later we visit Shechen Clinic and Hospice, a human- touring • Kathmandu Valley excursion • Visit to Buddhist Day 3: Kathmandu This morning we meet our itarian project that provides sustainable medical services monastery • Lunch with a Nepalese family in their home fellow travelers and Odysseys Tour Director for a to local residents; then tour Boudhanath, one of the • Patan’s traditional arts and handcrafts • Hindu shrine at briefing about the journey ahead. -

For a Cleaner and Greener Kathmandu Valley Kathmandu, Nepal May 11, 1994
OT FOR PUBLICATION CMC-1 WITHOUT WRITER'S CONSENT ] INSTITUTE OF CURRENT WORLD APA1RS For a cleaner and greener Kathmandu Valley Kathmandu, Nepal May 11, 1994 Mr. Peter B. Martin Institute of Currem World Aa'ah's 4 West Wheelock Street Hanover, New Hampshire 03755 USA Dear Peter, Touts, with trousers barely touching their ankles whisper in my ear,"Change mone)3 madam? Good price." The shops and restaurants blast out the best of the new and the old in Western mttsic as Madonna and Kansas mix with Nepali film songs in the sound waves of the nan'ow streets. "Come sit and drink tea with me madam. No? Maa-dam, it is breaking my heart!" a shopkeeper shouts as I pass by. That's me. A regular heart breaker. Welcome to Kathmandu. I am here for fmal arrangements for my visa into Bhutan, the largely unknown Himal Kingdom east of Nepal. Kathmandu seems relatively unchanged since my last Ssit in 1989. The narrow roads vind the same way" I remember. Shops overflow with the same goods; 'Free Tibet' T-shirts, Buddhist tankas delicately painted on canvas with colors from crushed stone, colorful woolen rugs, and silver jewelry inlaid with gem stones. The city itseff overflows with people and transport. Vehicle exhaust is black, thick and tickles the throat. I pity the individual standing behind a lonT when it shakes, grinds and exhales its choking fumes. I notice a number of motorists and pedestrians wearing small pieces of cloth across the nose and mouth, afftxed with string like a surgical mask. -

Understanding Children's Risk and Agency in Urban Areas
Understanding children’s risk and agency in urban areas for child-centred urban disaster risk reduction in Asia: Insights from Dhaka, Kathmandu, Manila and Jakarta Plan, IIED, ECO Nepal September 2013 Research Goal The research aims to generate an empirical evidence base on children’s risk and agency in urban areas that Plan International can use to inform the development of their Country Strategy Programmes (CSPs) and urban DRR programmes in Asia Four research questions • Which urban children are most at risk any why? • What are the factors that underpin urban children’s risk across the spectrum? • How can urban children participate in DRR as agents of change and resilience, and what are barriers and challenges they face in urban areas? • What the implications of this understanding for the development of child-centred urban DRR programmes? Research Method Qualitative methods • A total of 341 children participated in 16 focus groups (four in each city), including 183 girls (or 53.7 per cent) and 158 boys (or 46.3 per cent) between the ages of 8 and 20, achieving a relatively even gender balance and broad age distribution. • 3 children categories were targeted: street children, working children and squatter and ‘slum’ children. • Key informant interviews: to engage policymakers from relevant government departments and practitioners from local, national and international CSOs and development agencies as well as donors. Respondents were selected based on stakeholder mapping reports developed by consultants in each country, resulting in a -

Icrc Broucure Final 2068 Rewrok English.Indd
Pokhara H Nepalgunj Kathmandu H Chitwan Birgunj Delegation Janakpur Of ce Biratnagar H ICRC supported Physical Rehabilitation Center CONTACTS Green Pastures Hospital & Rehabilitation Center, HOW TO GET ASSISTANCE Hariyo Kharka, Pokhara. Tel: 061-431162/430099 People requiring physical rehabilitation services can contact Yerahity Rehabilitation Center, the Green Pastures Hospital or the Yerahity Rehabilitation Yerahity, Banasthali, Kathmandu. Centre directly. They can also contact their nearest Nepal Tel: 01-4880684/4880689 Red Cross Society Chapter / Sub-chapter or ICRC o!ces for or further information. Nepal Red Cross Society, Kalimati, Kathmandu. Tel: 01-4272761/4270650 Fax: 01-4271915 E-mail: [email protected] Web: www.nrcs.org or International Committee of the Red Cross, Physical Meen Bhawan, Naya Baneshwor, Kathmandu, Nepal. Tel: 01-4107285/4107279 Fax: 01-4107137 E-mail: [email protected] Web: www.icrc.org Rehabilitation ICRC Biratnagar ICRC Janakpur Tel: 021-470639/471358 Tel: 041-525126 Services ICRC Birgunj ICRC Nepalgunj Tel: 051-531690 Tel: 081-522262/525560 in Nepal ICRC Chitwan Tel: 056-523118 .28/002 06.2011 500 2010 T PHYSICAL REHABILITATION ICRC SUPPORT SERVICES PROVIDED Physicalrehabilitationhelpsrestorethefunctionalcapacityof In 2004, the ICRC in cooperation with the International Physical rehabilitation centres supported by the ICRC provide people who have lost limbs or injured spinal cords, allowing Nepal Fellowship (INF) started assisting the Green Pastures custom-made arti"cial limbs and support devices (orthoses), them to carry on as normal a life as possible. It includes both Hospital in Pokhara. The institution extended its support to crutches and wheelchairs designed according to the needs the provision of arti"cial limbs and other assistive devices the Yerahity Rehabilitation Centre run by the Nepal Army of the patients. -

Monthly OTP March 2019
Monthly OTP March 2019 ON-TIME PERFORMANCE AIRPORTS Contents On-Time is percentage of flights that depart or arrive within 15 minutes of schedule. Global OTP rankings are only assigned to all Airlines/Airports where OAG has status coverage for at least 80% of the scheduled flights. Regional Airlines Status coverage will only be based on actual gate times rather than estimated times. This may result in some airlines / airports being excluded from this report. If you would like to review your flight status feed with OAG please [email protected] MAKE SMARTER MOVES OAG Airport Monthly OTP – March 2019 Page 1 of 2 Home GLOBAL AIRPORTS – TOP 100 GLOBAL AIRPORT PERFORMANCE On-time performance GLOBAL AIRPORT PERFORMANCE On-time performance Airport Departures Rank No. flights Size Airport Departures Rank No. flights Size SIP Simferopol 98.4% 1 764 613 VOG Volgograd 92.7% 51 417 864 MSQ Minsk International Apt 97.7% 2 1,880 346 KYA Konya 92.7% 52 290 1,007 ARI Arica 97.6% 3 246 1,057 CJC El Loa Apt 92.7% 53 675 663 KRP Karup 97.6% 4 311 986 SUV Suva 92.6% 54 395 884 USN Ulsan 97.4% 5 351 939 UKB Osaka Kobe Airport 92.5% 55 1,241 451 TJM Tyumen 96.9% 6 747 622 TAE Daegu 92.3% 56 1,337 435 CCP Carriel Sur Intl Apt 96.6% 7 477 808 MBS Saginaw/Midland/Bay C. 92.3% 57 260 1,038 VQS Antonio Rivera Rodriguez Apt 96.6% 8 394 887 KCZ Kochi (JP) 92.2% 58 683 659 ROV Rostov 96.2% 9 980 518 ITO Hilo 92.2% 59 510 768 LDU Lahad Datu 96.1% 10 155 1,183 OKD Sapporo Okadama Apt 92.2% 60 358 932 TGU Toncontin Intl Apt 95.8% 11 567 733 LYH Lynchburg 92.1% 61 177 1,155 TTJ Tottori 95.5% 12 155 1,185 KSC Kosice 92.1% 62 193 1,130 YQU Grande Prairie 95.4% 13 371 912 TRS Trieste 92.0% 63 358 931 MRV Mineralnye Vody 95.3% 14 739 626 GUA La Aurora Intl Apt 91.9% 64 1,635 375 MGA Augusto C. -

Patterns in Pre-Lacteal Feeding Among Mothers in Urban Cambodia, Nepal, Senegal, and Tanzania
PATTERNS IN PRE-LACTEAL FEEDING AMONG MOTHERS IN URBAN CAMBODIA, NEPAL, SENEGAL, AND TANZANIA Assessment and Research on Child Feeding (ARCH) Alissa M. Pries, Khin Mengkheang, Indu Adhikary, Aminata Ndiaye, Margaret Benjamin, Mary Champeny, Bineti Vitta, Alison Feeley, Sandra L. Huffman Global Maternal Newborn Health Conference STUDY BACKGROUND • Pre-lacteal feeding and early use of breast-milk substitutes interferes with successful breastfeeding • Sales and promotion of breast-milk substitutes are increasing in low income countries • Heath facility-based marketing can have a negative impact on early breastfeeding practices and duration of breastfeeding Promoting breastmilk substitutes within the health system can negatively impact breastfeeding, thereby threatening infant and young child nutrition and health. RESEARCH OBJECTIVES • Estimate the prevalence of promotional practices occurring within the health system for breast-milk substitutes – Commercial advertising and display of product branding – Recommendations from health professionals – Free samples and branded gifts • Document breastfeeding support and counseling provided in health facilities • Document early breastfeeding practices and consumption of breast- milk substitutes among newborns (pre-lacteal feeding) RESEARCH DESIGN • Structured interviews among mothers discharged after delivery in Phnom Penh, Kathmandu Valley, Dakar Department, and Dar es Salaam • Cross-sectional, health facility-based survey • Sample size of 280 mothers in each site to detect 10% promotion prevalence -

Map of South Asia
UZ BE Kashi 60° 65° K 75° 80° 85° 90° 95° IS TAJIKISTAN TURKMENISTAN T D A N u c h a nb Hotan SOUTH ASIA e 35° Gilgit 35° Herāt Kābul Chinese Jammu Line Indian Line AFGHANISTAN Peshawar In and du s ād CHINA Islāmāb i Kashmir ind walp Kandahar- Ra Gujranwala Zhob Lahore Amritsar 30° Simla Quetta 30° Multan Ludhiana Chandigarh N Lhasa Indian Line A j utle Yarlung T Bahawalpur S Delhi N Chinese Line I S (Brahmaputra) Xigazê phu P A K Bareilly im Sukkur New Delhi G G E Mount Everest h s Y u a Dibrugarh h 8848 m T Larkana d a n n a I m g P Itanagar a es g r u h A t Dadu n a Kathmandu pu Jaipur a r L BHUTAN a Lucknow a hm Turbad Bra Jodhpur al Gorakhpur Hyderabad mb Kanpur Dispur ha Ga 25° C Gwalior nges Shillong Kota P 25° Karachi V a Udaipur a tn (B r a Imphal a a n na BANGLADESH a si A Gandhinagar ra g Aizawl s) art G Ahmadabad Bhopal Jabalpur Kolkata Dhaka ala ulf o f Kutch Indore Jamshedpur (Calcutta) Khulna Chittagong Vadodara Raurkela a s Mandalay Rajkot Narmad Mahanadi Kharagpur ge n a t M e G a outh of th h Surat Nagpur Raipur b Cuttack Jaipur MYANMAR m 20° a Daman h 20° f K Silvassa f o Bhubaneswar Sittwe Gul INDIA Kalyan Go vari Mumbai Pune da (Bombay) Bh im Hyderabad a Vishakhapatnam A r a b i a Kolhapur Vijayawada Kakinada n S e a K g a l rishna B a y o f B e n Belgaum Guntur Machilipatnam 15° Panaji Bellary 15° A n Bangalore Chennai d Mangalore (Madras) a Vellore Port Blair m Mysore Pondicherry Andaman L a L Kozhikode Salem Islands a a (INDIA) n k (Calicut) s 10° ( K c I h 10° N S a a Madurai D v c d I a e A w r a Cochin Jaffna a ) e a t t d Tuticorin e i p i r a Trincomalee v Trivandrum n Nicobar an e f M Negombo SRI LANKA Islands lf o Gu (INDIA) Kandy Sri Jayewardenepura-Kotte S e Galle 5° MALDIVES a Matara 5° Maldive The boundaries and names shown and the designations used Male on this map do not imply official endorsement or acceptance Islands by the United Nations. -

Foreign Aid in Waste Management: a Case of Kathmandu, Nepal * Mohan B
Originally published by Elsevier in Habitat International 49 (2015) 393e402. DOI: 10.1016/j.habitatint.2015.06.010 Foreign aid in waste management: A case of Kathmandu, Nepal * Mohan B. Dangi , Erica Schoenberger, John J. Boland Department of Geography and Environmental Engineering, The Johns Hopkins University, 3400 N. Charles Street, Baltimore, MD 21218, United States Abstract: Four decades of reorganization of waste management in Kathmandu, Nepal funded by foreign aid failed to provide adequate services and led to the return of riverbank waste disposal by 1994. To assess the results of foreign aid in waste management in Kathmandu from 1970 to 2010, the researchers utilized qualitative and field methods and examined three major international projects sponsored by the gov-ernments of Germany, India, and Japan. Results suggest that German aid was too technical, undermining municipal capacity and burdening the city with a second waste disposal institution while failing to sustain its own infrastructure. The Indian project lacked focus and follow up programs and encumbered a poor country with outdated equipment that did not meet the local needs. Japanese aid depended on wrong assumptions, stressing costly landfilling that employed heavy machinery and upgraded equip-ment inappropriate for local conditions. The study recommends that Nepal institute bottom-up and participatory style of waste management that identifies where the resources will come from, who will manage them, and how they will be sustained. Keywords: Municipal solid waste, Solid waste management, Foreign aid in waste management, Effectiveness of foreign aid in Kathmandu, Nepal 1. Introduction transfer to these local authorities. Nevertheless, even as the local government assumed public solid waste disposal in the city, the In Nepal, only three municipalities have some sort of formal deposition of solid wastes into the Bagmati and the Bishnumati management of municipal solid waste (MSW). -

Asian Cities Climate Resilience Understanding Children's Risk And
Asian Cities Climate Resilience WoRKING PAPER SERIES 6: 2014 Understanding children’s risk and agency in urban areas and their implications for child- centred urban disaster risk reduction in Asia: Insights from Dhaka, Kathmandu, Manila and Jakarta By DoNALD BRoWN AND DAvID DoDmAN Understanding children’s risk and agency in urban areas and their implications for child- centred urban disaster risk reduction in Asia Asian Cities Climate Resilience Working Paper Series This working paper series aims to present research outputs around the common theme of urban climate resilience in Asia. It serves as a forum for dialogue and to encourage strong intellectual debate over concepts relating to urban resilience, results from the ground, and future directions. The series is also intended to encourage the development of local research capacity and to ensure local ownership of outputs. The series has arisen out of the Asian Cities Climate Change Resilience Network (ACCCRN, www.acccrn.org), an initiative funded by the Rockefeller Foundation. The series is intended to present research in a preliminary form for feedback and discussion. Readers are encouraged to provide comments to the authors whose contact details are included in each publication. About Plan Plan has been working for, and with, children for more than 75 years. With no religious, political or governmental affiliations, we works in 50 developing countries across Africa, Asia and the Americas to promote child rights and lift millions of children out of poverty. We focus on the inclusion, education, and protection of the most marginal- ised children, working in partnership with communities, local and national government and civil society. -

Himalayan Kingdoms: Nepal & Bhutan
HIMALAYAN KINGDOMS: NEPAL & BHUTAN September 19-October 3, 2017 15 days from $4,797 total price from Boston ($4,195 air & land inclusive plus $602 airline taxes and fees) This tour is provided by Odysseys Unlimited, six-time honoree Travel & Leisure’s World’s Best Tour Operators award. An Exclusive Small Group Tour for Alumni, Family, & Friends of Dartmouth College Featuring Dr. Kenneth Bauer, Program Manager of Human Development, Dickey Center at Dartmouth College Dear Alumni, Family, and Friends of Dartmouth, Journey with us to the Himalayan kingdoms of Nepal and Bhutan! Discover fabled lands tucked high up in the Himalayas, one steeped in Hindu culture and tradition, the other in Buddhism. Dr. Kenneth Bauer of Dartmouth College will enrich our tour with lectures and commentary. Our tour begins in Kathmandu, where we visit Swayambhunath, an iconic Buddhist temple and pilgrimage site. Continuing on to Patan, known for its handcrafts, we take a walking tour of Patan Durbar Square, a UNESCO site. We travel through the beautiful Kathmandu Valley – home to seven UNESCO sites – to Kopan Monastery, then enjoy a small group highlight: lunch in a local family’s home. Next we visit Boudhanath, Nepal’s largest stupa and a UNESCO site, and Pashu- patinath Temple, Nepal’s oldest Hindu shrine. After touring ancient Bhaktapur, we enjoy a scenic drive to hilltop Nagarkot. Flying to Thimphu, Bhutan, we tour the city and enjoy a home-hosted dinner. We raise prayer flags at Dochula Pass and take an exhilarating river rafting trip. Then we visit beautiful Punakha Dzong monastery and tour historic Paro. -

URBAIR. Urban Air Quality Management Strategy in Asia. DKI
Urban Air Quality Management Strategy in Asia RECEIVED DEC 0 5 m QSTl DKI JAKARTA City Specific Report DISTRIBUTION OF THIS DOCUMENT IS UNLIMITED Prepared under contract from foreign sales prohibited The World Bank Asia Technical Division Instituut voor Milieuvraagstukken The Norwegian Institute for Air Research Vrije Universiteit, De Boelelaan 1115, NILU P.O.Box 100, N-2007 Kjeller, Norway 1081 HV Amsterdam, The Netherlands DISCLAIMER Portions of this document may be illegible electronic image products. Images are produced from the best available original document. NTLU : OR 19/96 REFERENCE : 0-92117 DATE MARCH 1996 ISBN 82-425-0756-2 URBA1R Urban Air Quality Management Strategy in Asia DKI JAKARTA City Specific Report Prepared by Knut Erik Gr0nskei, Frederick Gram, Leif Otto Hagen and Steinar Larssen, Norwegian Institute for Air Research, Kjeller, Norway Huib Jansen and Xander Olsthoorn, Instituut voor Milieuvraagstukken (IVM) Vrije Universiteit, Amsterdam, the Netherlands Moestikahadi Soedomo Dpt. of Environment Engineering, Inst, of Technology Bandung Umar F. Achmadi Faculty of Public Health, Univ. of Indonesia Instituut voor Milieuvraagstukken The Norwegian Institute for Air Research Vrije Universiteit, De Boelelaan 1115, iNILUl P.O.Box 100, N-2007 Kjeller, Norway 1081 HV Amsterdam, The Netherlands Notice This report from the URBAIR project conducted under the Metropolitan Environment Improvement Program of the World Bank, ASTEN Division, is the version produced by the project consultants (Norwegian Institute for Air Research and Institute for Environmental Studies in Amsterdam) for the World Bank. The World Bank publishes the official version of this report. The contents is basically the same, but the layout is somewhat different. -
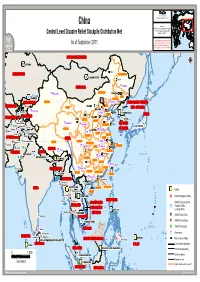
Central Level Disaster Relief Stockpile Distribution Net UNJLC - UNSDI-T
FF II CC SS SS Field Information and China Coordination Support Section Sources: UNHCR, Global Insight digital mapping © 1998 Europa Technologies Ltd. Central Level Disaster Relief Stockpile Distribution Net UNJLC - UNSDI-T The boundaries and names shown and the designations used on this As of September 2011 map do not imply official endorsement or acceptance by the United Nations. China_CLDRS_DistribNet_A3LC.WOR RUSSIAN FEDERATION ASTANAASTANA !! Hailar KAZAKHSTAN HEILONGJIANGHEILONGJIANG !! ULAANBAATARULAANBAATAR !! !! !! Qiqihar MONGOLIA CCC!! HarbinHarbin NEIMENGGUNEIMENGGU !! CC!! UrumshiUrumshi Changchung !! !! Jilin !! AlmatyAlmaty CCC !! JILINJILINJILIN BISHKEKBISHKEK LIAONINGLIAONING XINJIANGXINJIANG HEBEIHEBEI ShenyangShenyangC!!!! Fushun !! CC !! KYRGYZSTAN CCC DEMOCRATIC PEOPLE'S !! !! Anshan !! Hohhot !! BEIJINGBEIJING !! BEIJINGBEIJING !! BEIJINGBEIJING !! BEIJINGBEIJING !! BEIJINGBEIJING Baotou !! BEIJINGBEIJING UZBEKISTAN OshOsh GANSUGANSU !! REP. OF KOREA !! !! BEIJINGBEIJINGCC !! Tangshan !! KashgarKashgar CCC DushanbeDushanbe CC !! DushanbeDushanbe CC TienjinTienjin !! !! CCC TienjinTienjinCC !! Dalian !! P'YONGYANGP'YONGYANG !! CCC !! !! Yinchuan TIANJINTIANJIN JAPAN !! Shijiazhuang !! !! !! !! TAJIKISTAN Taiyuan !! TURKMENISTAN !! Xining NINGXIANINGXIA !! SEOULSEOUL NINGXIANINGXIA SHANXISHANXI KunduzKunduz !! !! Jinan Mazar-i-SharifMazar-i-Sharif GolmudGolmud Mazar-i-SharifMazar-i-Sharif CCC !! LanzhouLanzhou SHANDONGSHANDONG !! Qingdao REPUBLIC CC SHANDONGSHANDONG !! MaimanaMaimana KabulKabul