2015 Fracture of the Nasal Bones in a Horse by Nathalie
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CASE REPORT Injuries Following Segway Personal
UC Irvine Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health Title Injuries Following Segway Personal Transporter Accidents: Case Report and Review of the Literature Permalink https://escholarship.org/uc/item/37r4387d Journal Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health, 16(5) ISSN 1936-900X Authors Ashurst, John Wagner, Benjamin Publication Date 2015 DOI 10.5811/westjem.2015.7.26549 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California CASE REPORT Injuries Following Segway Personal Transporter Accidents: Case Report and Review of the Literature John Ashurst DO, MSc Conemaugh Memorial Medical Center, Department of Emergency Medicine, Benjamin Wagner, DO Johnstown, Pennsylvania Section Editor: Rick A. McPheeters, DO Submission history: Submitted April 20, 2015; Accepted July 9, 2015 Electronically published October 20, 2015 Full text available through open access at http://escholarship.org/uc/uciem_westjem DOI: 10.5811/westjem.2015.7.26549 The Segway® self-balancing personal transporter has been used as a means of transport for sightseeing tourists, military, police and emergency medical personnel. Only recently have reports been published about serious injuries that have been sustained while operating this device. This case describes a 67-year-old male who sustained an oblique fracture of the shaft of the femur while using the Segway® for transportation around his community. We also present a review of the literature. [West J Emerg Med. 2015;16(5):693-695.] INTRODUCTION no parasthesia was noted. In 2001, Dean Kamen developed a self-balancing, zero Radiograph of the right femur demonstrated an oblique emissions personal transportation vehicle, known as the fracture of the proximal shaft of the femur with severe Segway® Personal Transporter (PT).1 The Segway’s® top displacement and angulation (Figure). -

Management of the Traumatized Airway
CLINICAL CONCEPTS AND COMMENTARY Jerrold H. Levy, M.D., F.A.H.A., F.C.C.M., Editor Management of the Traumatized Airway Uday Jain, M.D., Ph.D., Maureen McCunn, M.D., M.I.P.P., Charles E. Smith, M.D., Jean-Francois Pittet, M.D. IRWAY injury is a major cause of early death in Anatomy of Trauma to the Airway 1,2 A trauma. The incidence of traumatic airway injuries is Airway injuries can be divided into three types: maxillofa- 3,4 low, although it is recently increasing. In contrast, mortal- cial, neck, and laryngeal injury. ity due to traumatic airway injuries is high, in part, because of associated injuries to other organs, which are present in Maxillofacial Trauma about one half of the cases of blunt or penetrating airway Blunt or penetrating trauma to the face can affect the max- 1,2 trauma. Patients with a significant injury severity (scored illary/mandibular or mid-facial region and extend intra- 5 on a scale of 0 to 75, which accounts for the most severely cranially12–14 (table 1). Maxillofacial trauma can result in traumatized body systems) have a higher predicted mortality. life-threatening airway and hemorrhage problems and lead In a retrospective review of 12,187 civilian patients treated to significant ocular, nasal, and jaw dysfunction. Bleeding at a regional trauma center in Toronto from 1989 to 2005, may complicate airway management. Swallowing of blood 36 patients (0.3%) had blunt airway trauma (injury sever- clears the airway and is facilitated with the patient in the sit- ity score, 33; mortality, 36%) and 68 patients (0.6%) had ting position. -

Clinical Review: Facial Fracture
CLINICAL Fracture, Facial REVIEW Indexing Metadata/Description › Title/condition: Fracture, Facial › Synonyms: Facial fracture › Anatomical location/body part affected: Face/mandible, orbit floor, maxilla, zygomatic arch, nose, cranial base, occiput › Area(s) of specialty: Orthopedic Rehabilitation › Description: Traumatic fractures of the midface and jaw, excluding skull/cranial fractures • Head and neck trauma is increasing in frequency in modern combat and is thought to be due to the increased use of improvised explosive devices and the increased likelihood of surviving severe wounds(8) › ICD-10 codes: • S07.0 crushing injury of face • S02.1 fracture of base of skull • S02.11 fracture of occiput • S02.2 fracture of nasal bones • S02.3 fracture of orbital floor • S02.4 fracture of malar, maxillary, and zygoma bones • S02.41 LeFort fracture • S02.6 fracture of mandible • S02.9 fracture of unspecified skull and facial bones (ICD codes are provided for the reader’s reference only, not for billing purposes) › Reimbursement: Reimbursement for therapy will depend on insurance contract coverage; no special agencies or specific issues regarding reimbursement have been identified for this condition. If the patient is an athlete with multiple facial fractures, a custom-made face mask might be required prior to return to athletic participation. Unfortunately, insurance might not cover the device, making it cost prohibitive and thus forcing an athlete to return to athletic competition at a much later time(1) Author › Presentation/signs and symptoms -

Nasal Bone Fractures and the Use of Radiographic Imaging: an Otolaryngologist Perspective Running Title: Nasal Bone Fractures and Radiology
Journal Pre-proof Nasal Bone Fractures and the Use of Radiographic Imaging: An Otolaryngologist Perspective Running Title: Nasal bone fractures and Radiology Edward Westfall, MD1,2 Benton Nelson, MD1,2 Dominic Vernon, MD1,2 Mohamad Z. Saltagi, MD1,2 Avinash V. Mantravadi, MD1,2 Cecelia Schmalbach, MD1,2 Jonathan Y. Ting, MD, MS, MBA1,2 Taha Z. Shipchandler, MD1,2 Author Affiliations: 1Department of Otolaryngology—Head and Neck Surgery 2Indiana University School of Medicine Corresponding Author: Taha Z. Shipchandler, MD, FACS Division Director – FacialJournal Plastic, Aesthetic, Pre-proof & Reconstructive Surgery Associate Professor Vice-Chair and Residency Director Department of Otolaryngology—Head and Neck Surgery, Indiana University Health Physicians, Indiana University School of Medicine 1130 W. Michigan Street, Suite 400, Indianapolis, IN 46202 Telephone: 317-278-1258 Fax: 317-274-8285 [email protected] Financial disclosures: none Authors disclose no conflict of interest ____________________________________________________ This is the author's manuscript of the article published in final edited form as: Westfall, E., Nelson, B., Vernon, D., Saltagi, M. Z., Mantravadi, A. V., Schmalbach, C., … Shipchandler, T. Z. (2019). Nasal bone fractures and the use of radiographic imaging: An otolaryngologist perspective. American Journal of Otolaryngology, 102295. https://doi.org/10.1016/j.amjoto.2019.102295 Journal Pre-proof Abstract Objective: To determine radiologic preferences of practicing otolaryngologists regarding isolated nasal bone fractures. Study Design: An 8-question survey on isolated nasal bone fractures was designed. Setting: Surveys were sent to all otolaryngology residency program directors for distribution among residents and faculty. Additional surveys were distributed to private practice otolaryngology groups. Subjects and Methods: Practicing academic (residents & faculty) and private-practice otolaryngologists. -

Improving the Quality of Assessment and Management of Nasal Trauma in a Major Trauma Centre (MTC): Queen Elizabeth Hospital, Birmingham
Open access Quality improvement report BMJ Open Qual: first published as 10.1136/bmjoq-2019-000632 on 27 November 2019. Downloaded from Improving the quality of assessment and management of nasal trauma in a major trauma centre (MTC): Queen Elizabeth Hospital, Birmingham Apoorva Khajuria ,1 Max Sallis Osborne ,1 Lisha McClleland,1 Sandip Ghosh2 To cite: Khajuria A, Osborne MS, ABSTRACT no specific guidelines to provide a standard McClleland L, et al. Improving Background Nasal fractures present in 39% of patients of care for nasal fracture patients. Therefore, the quality of assessment and with facial trauma. These patients are assessed in the management of nasal trauma junior doctors with little prior ENT experi- emergency department followed by outpatient review in a major trauma centre (MTC): ence may find themselves perplexed when Queen Elizabeth Hospital, in the senior house officer- led emergency ear, nose and assessing such injuries. Birmingham. BMJ Open Quality throat (ENT) clinic. Inadequate treatment of nasal trauma Violent crime and antisocial behaviour in can result in debilitating functional and aesthetic problems. 2019;8:e000632. doi:10.1136/ Birmingham is on the rise; we found assault bmjoq-2019-000632 Inexperienced junior doctors may be apprehensive in and violence the most common causes of assessing nasal trauma resulting in time pressured clinics 1 ► Additional material is and suboptimal management. nasal injuries in our patient cohort. The ENT published online only. To view Measures A retrospective review of clinical noting over senior house officer (SHO) on-call would please visit the journal online 3 months was carried out to gauge the extent of the book nasal trauma patients into the SHO- (http:// dx. -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more -
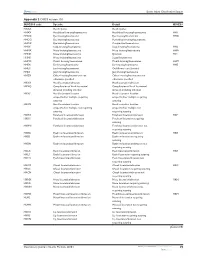
Appendix 2 OSICS Version 10.1 (Continued)
Dovepress Sports Injury Classification System Appendix 2 OSICS version 10.1 OSICS10 code Specific Detail OSICS9 HXXX Head injuries Head injuries HHXX Head/facial bruising/haematoma Head/facial bruising/haematoma HH1 HHOX Eye bruising/haematoma Eye bruising/haematoma HHO HHOO Eye bruising/haematoma Periorbital bruising/haematoma HHOC Eye bruising/haematoma Conjunctival haematoma HHSX Scalp bruising/haematoma Scalp bruising/haematoma HHS HHNX Nose bruising/haematoma Nose bruising/haematoma HHN HHNE Nose bruising/haematoma Epistaxis HV1 HHNS Nose bruising/haematoma Septal haematoma HHMX Mouth bruising/haematoma Mouth bruising/haematoma HHM HHEX Ear bruising/haematoma Ear bruising/haematoma HHE HHEC Ear bruising/haematoma Cauliflower ear (chronic) HHJX Jaw bruising/haematoma Jaw bruising/haematoma HHZX Other bruising/haematoma not Other bruising/haematoma not otherwise specified otherwise specified HKXX Head laceration/abrasion Head laceration/abrasion HKXQ Complication of head laceration/ Complication of head laceration/ abrasion including infection abrasion including infection HKXS Head laceration location Head laceration location unspecified/or multiple requiring unspecified/or multiple requiring suturing suturing HKXN Head laceration location Head laceration location unspecified/or multiple not requiring unspecified/or multiple not suturing requiring suturing HKHX Forehead laceration/abrasion Forehead laceration/abrasion HKF HKHS Forehead laceration/abrasion Forehead laceration requiring suturing HKHN Forehead laceration/abrasion Forehead -

JISHANT Assessment of Nasal Trauma
Journal of the International Society of Head and Neck Trauma (ISHANT) Invited review Assessment of nasal trauma Dr SUMIT SHARMA. MBBS (ENT-Honors) (KGMC-Lko), M.S. (KGMC-Lko), Assistant Professor, Department of E.N.T., Mayo Institute of Medical Sciences, Barabanki, India [email protected] Received July 2016. Accepted following peer review September 2016. Published September 2016 JISHANT 2016:7 Introduction The assessment of nasal injuries accidents, sports injuries and during requires a delicate balance of history physical confrontation. Because of this taking and clinical examination. they are twice as more common in males Management of these injuries must take than in females. The nose is the most into consideration both functional and prominent feature of the face and has cosmetic aspects. Although not an little protection or support. exhaustive review of the literature this Nasal bones are very brittle and can paper discusses key aspects of be broken easily with trivial impacts. assessment with observations from my Low impact forces of 30G can be own experience. sufficient to result in fracture, compared Pathophysiology to the supraorbital rim which requires a force of 200G on impact (2). The ease Nasal bone fractures are the with which the nose is broken may help commonest facial fracture and make up absorb the energy of impact and offer to 39% of all maxillofacial injuries some protection to the brain. Thus it has (1).These types of fractures are mainly been suggested this acts as a protective seen in road traffic mechanism (3). Journal of the International Society of Head and Neck Trauma (ISHANT) From the structural point of view the side of the point of impact. -

Comparison of Ultrasonography and Conventional Radiography in the Diagnosis of Nasal Fractures
ORIGINAL ARTICLE Comparison of Ultrasonography and Conventional Radiography in the Diagnosis of Nasal Fractures Oliver Thiede, MD; Jan-Hendrik Krömer, MD; Claudia Rudack, MD; Wolfgang Stoll, MD; Nani Osada, MD; Frank Schmäl, MD Objective: To compare the diagnostic findings of ul- The results were analyzed by various statistical testing trasonography and radiography in nasal fractures. methods (for sensitivity, specificity, positive and nega- tive predictive value, and accuracy). Design and Main Outcome Measures: In this pro- spective study, 63 patients (23 female and 40 male; mean Results: Assessment of the lateral nasal walls revealed age, 26.8 years) with clinical signs of a nasal bone frac- that ultrasonography was statistically superior (P=.04) ture were investigated. All patients underwent radiogra- to radiography. In contrast, assessment of the nasal dor- phy (lateral view of the nose plus occipitomental view) sum showed radiography to be statistically superior and ultrasonography (10-MHz ultrasound scanner) of the (P=.01) to ultrasonography. Assessment of the nasal pyra- nasal dorsum and the lateral nasal walls and a clinical mid revealed no statistical difference between radiogra- examination by 2 consultants. Thirty-six patients under- phy and ultrasonography (P=.91). went nasal fracture reduction. Two radiographs and 3 ul- trasound images of each patient were analyzed by 2 Conclusion: In assessment of the nasal pyramid, ultra- experienced readers at different times. After assessing the sonography can be considered an alternative to radiog- nasal dorsum and lateral nasal walls in radiographs and raphy, with equivalent diagnostic performance. ultrasound images, they decided whether the nose was fractured or not or whether the results were uncertain. -

Coding Companion for Plastics/Dermatology a Comprehensive Illustrated Guide to Coding and Reimbursement
ATPR.book Page 1 Thursday, N ovem ber 15, 2012 3:09 PM Coding Companion for Plastics/Dermatology A comprehensive illustrated guide to coding and reimbursement 2013 ATPR.book Page i Thursday, N ovem ber 15, 2012 3:09 PM Contents Getting Started with Coding Companion .............................i Arteries/Veins...................................................................365 Skin .....................................................................................1 Lips .................................................................................368 Nails ..................................................................................30 Vestibule of Mouth ..........................................................381 Pilonidal Cyst.....................................................................34 Tongue............................................................................384 Repair ................................................................................35 Palate/Uvula ....................................................................388 Destruction......................................................................131 Abdomen ........................................................................402 Breast ..............................................................................144 Genitalia..........................................................................403 General Musculoskeletal ..................................................167 Extracranial Nerves ..........................................................407 -

Surgical Management of Facial Fractures
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY SURGICAL MANAGEMENT OF MAXILLOFACIAL TRAUMA Prabhat Bhama, Mack Cheney Maxillofacial trauma is typically caused by The bony fragments may remain anatomi- blunt trauma due to interpersonal violence, cally reduced or can become displaced moving vehicle accidents (MVA), falls, or depending on the mechanism of injury and sporting activities 1. Because these activi- muscular forces acting on the fragments. ties transcend cultural and geographic The degree of anatomic disruption of frac- borders, maxillofacial trauma remains a tured segments is referred to as displace- global health issue. Technological advan- ment. Blood flow to the fracture site re- ces have resulted in faster methods of sults in callus formation over time follow- transportation and with these developments ed by mineralisation of the callus if the maxillofacial trauma has become a major fracture site is immobilised. public health issue also in the developing world. Although management of the air- Malunion occurs when fracture segments way and cardiopulmonary system take pre- are not reduced and the bone heals in an cedence, facial injuries demand attention anatomically incorrect position 3. In the because of their potential for long-term face, this can cause significant disfigure- impact on quality of life (QOL) and the ment and disability. patient’s psychological wellbeing 2 as the face houses many vital structures neces- Disfigurement occurs because the skin soft sary for sight, hearing, speech, olfaction, tissue envelope (SSTE) of the face is and mastication. dependent upon the underlying maxillo- facial skeletal framework. Anatomic per- turbations of the bony framework from Pathophysiology maxillofacial trauma manifest as abnormal appearances of the SSTE. -

Chapter 24 ORBITAL TRAUMA and NASOETHMOID FRACTURES
Orbital Trauma and Nasoethmoid Fractures Chapter 24 ORBITAL TRAUMA AND NASOETHMOID FRACTURES † ‡ § CARLOS AYALA, MD, FACS*; BRIAN GROAT, MD ; LISA MIHORA, MD ; AND DEMETRIO AGUILA, MD, FACS INTRODUCTION ORBITAL ANATOMY Eyelids and Globe Bony Orbit Naso-Orbito-Ethmoid Complex Facial Buttress Anatomy OCULAR TRAUMA PATIENT EVALUATION Imaging History Physical Examination ORBITAL, MID-FACE, AND NASO-ORBITO-ETHMOID FRACTURES Orbital Fractures Mid-Face Fractures Zygomaticomaxillary Complex Fractures Naso-Orbito-Ethmoid Fractures OTHER ORBITAL INJURIES AND THEIR MANAGEMENT Foreign Bodies Open Globe Injury Closed Globe Injury Adnexal and Soft Tissue Injury Chemical Injuries Sympathetic Ophthalmia SUMMARY CASE PRESENTATIONS Case 24-1: Motor Vehicle Accident Case 24-2: Open Globe and Facial Trauma From Improvised Explosive Device Case 24-3: Improvised Explosive Device Fragment in Right Eye * Lieutenant Colonel (Retired), Medical Corps, US Air Force; Ayala ENT & Facial Plastic Surgery, 800 East Dove Avenue, Suites F & G, McAllen, Texas 78504; formerly, Chief, Facial Plastic Surgery and ENT, Nellis Air Force Base † Major, Medical Corps, US Air Force; Ophthalmologist, Live Oak Ophthalmology, 1915 South 17th Street, Suite 101, Wilmington, North Carolina 28401; formerly, Chief of Ophthalmology, Mike O’Callaghan Federal Medical Center, Nellis Air Force Base ‡ Lieutenant Colonel, Medical Corps, US Air Force Reserve; Ophthalmologist, Oculofacial Plastics, Barnes Health, 14416 West Meeken Boulevard, Building C, No. 201, Sun City West, Arizona 85375; formerly,