WASP: a Software Package for Correctly Characterizing the Topological Development of Ribbon Structures Zachary Sierzega1,3, Jef Wereszczynski1* & Chris Prior2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complete Invariant Graphs of Alternating Knots
Complete invariant graphs of alternating knots Christian Soulié First submission: April 2004 (revision 1) Abstract : Chord diagrams and related enlacement graphs of alternating knots are enhanced to obtain complete invariant graphs including chirality detection. Moreover, the equivalence by common enlacement graph is specified and the neighborhood graph is defined for general purpose and for special application to the knots. I - Introduction : Chord diagrams are enhanced to integrate the state sum of all flype moves and then produce an invariant graph for alternating knots. By adding local writhe attribute to these graphs, chiral types of knots are distinguished. The resulting chord-weighted graph is a complete invariant of alternating knots. Condensed chord diagrams and condensed enlacement graphs are introduced and a new type of graph of general purpose is defined : the neighborhood graph. The enlacement graph is enriched by local writhe and chord orientation. Hence this enhanced graph distinguishes mutant alternating knots. As invariant by flype it is also invariant for all alternating knots. The equivalence class of knots with the same enlacement graph is fully specified and extended mutation with flype of tangles is defined. On this way, two enhanced graphs are proposed as complete invariants of alternating knots. I - Introduction II - Definitions and condensed graphs II-1 Knots II-2 Sign of crossing points II-3 Chord diagrams II-4 Enlacement graphs II-5 Condensed graphs III - Realizability and construction III - 1 Realizability -

Notes on the Self-Linking Number
NOTES ON THE SELF-LINKING NUMBER ALBERTO S. CATTANEO The aim of this note is to review the results of [4, 8, 9] in view of integrals over configuration spaces and also to discuss a generalization to other 3-manifolds. Recall that the linking number of two non-intersecting curves in R3 can be expressed as the integral (Gauss formula) of a 2-form on the Cartesian product of the curves. It also makes sense to integrate over the product of an embedded curve with itself and define its self-linking number this way. This is not an invariant as it changes continuously with deformations of the embedding; in addition, it has a jump when- ever a crossing is switched. There exists another function, the integrated torsion, which has op- posite variation under continuous deformations but is defined on the whole space of immersions (and consequently has no discontinuities at a crossing change), but depends on a choice of framing. The sum of the self-linking number and the integrated torsion is therefore an invariant of embedded curves with framings and turns out simply to be the linking number between the given embedding and its small displacement by the framing. This also allows one to compute the jump at a crossing change. The main application is that the self-linking number or the integrated torsion may be added to the integral expressions of [3] to make them into knot invariants. We also discuss on the geometrical meaning of the integrated torsion. 1. The trivial case Let us start recalling the case of embeddings and immersions in R3. -

Gauss' Linking Number Revisited
October 18, 2011 9:17 WSPC/S0218-2165 134-JKTR S0218216511009261 Journal of Knot Theory and Its Ramifications Vol. 20, No. 10 (2011) 1325–1343 c World Scientific Publishing Company DOI: 10.1142/S0218216511009261 GAUSS’ LINKING NUMBER REVISITED RENZO L. RICCA∗ Department of Mathematics and Applications, University of Milano-Bicocca, Via Cozzi 53, 20125 Milano, Italy [email protected] BERNARDO NIPOTI Department of Mathematics “F. Casorati”, University of Pavia, Via Ferrata 1, 27100 Pavia, Italy Accepted 6 August 2010 ABSTRACT In this paper we provide a mathematical reconstruction of what might have been Gauss’ own derivation of the linking number of 1833, providing also an alternative, explicit proof of its modern interpretation in terms of degree, signed crossings and inter- section number. The reconstruction presented here is entirely based on an accurate study of Gauss’ own work on terrestrial magnetism. A brief discussion of a possibly indepen- dent derivation made by Maxwell in 1867 completes this reconstruction. Since the linking number interpretations in terms of degree, signed crossings and intersection index play such an important role in modern mathematical physics, we offer a direct proof of their equivalence. Explicit examples of its interpretation in terms of oriented area are also provided. Keywords: Linking number; potential; degree; signed crossings; intersection number; oriented area. Mathematics Subject Classification 2010: 57M25, 57M27, 78A25 1. Introduction The concept of linking number was introduced by Gauss in a brief note on his diary in 1833 (see Sec. 2 below), but no proof was given, neither of its derivation, nor of its topological meaning. Its derivation remained indeed a mystery. -
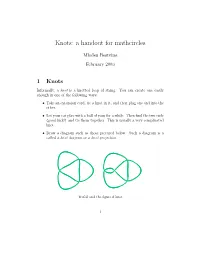
Knots: a Handout for Mathcircles
Knots: a handout for mathcircles Mladen Bestvina February 2003 1 Knots Informally, a knot is a knotted loop of string. You can create one easily enough in one of the following ways: • Take an extension cord, tie a knot in it, and then plug one end into the other. • Let your cat play with a ball of yarn for a while. Then find the two ends (good luck!) and tie them together. This is usually a very complicated knot. • Draw a diagram such as those pictured below. Such a diagram is a called a knot diagram or a knot projection. Trefoil and the figure 8 knot 1 The above two knots are the world's simplest knots. At the end of the handout you can see many more pictures of knots (from Robert Scharein's web site). The same picture contains many links as well. A link consists of several loops of string. Some links are so famous that they have names. For 2 2 3 example, 21 is the Hopf link, 51 is the Whitehead link, and 62 are the Bor- romean rings. They have the feature that individual strings (or components in mathematical parlance) are untangled (or unknotted) but you can't pull the strings apart without cutting. A bit of terminology: A crossing is a place where the knot crosses itself. The first number in knot's \name" is the number of crossings. Can you figure out the meaning of the other number(s)? 2 Reidemeister moves There are many knot diagrams representing the same knot. For example, both diagrams below represent the unknot. -

Computing the Writhing Number of a Polygonal Knot
Computing the Writhing Number of a Polygonal Knot ¡ ¡£¢ ¡ Pankaj K. Agarwal Herbert Edelsbrunner Yusu Wang Abstract Here the linking number, , is half the signed number of crossings between the two boundary curves of the ribbon, The writhing number measures the global geometry of a and the twisting number, , is half the average signed num- closed space curve or knot. We show that this measure is ber of local crossing between the two curves. The non-local related to the average winding number of its Gauss map. Us- crossings between the two curves correspond to crossings ing this relationship, we give an algorithm for computing the of the ribbon axis, which are counted by the writhing num- ¤ writhing number for a polygonal knot with edges in time ber, . A small subset of the mathematical literature on ¥§¦ ¨ roughly proportional to ¤ . We also implement a different, the subject can be found in [3, 20]. Besides the mathemat- simple algorithm and provide experimental evidence for its ical interest, the White Formula and the writhing number practical efficiency. have received attention both in physics and in biochemistry [17, 23, 26, 30]. For example, they are relevant in under- standing various geometric conformations we find for circu- 1 Introduction lar DNA in solution, as illustrated in Figure 1 taken from [7]. By representing DNA as a ribbon, the writhing number of its The writhing number is an attempt to capture the physical phenomenon that a cord tends to form loops and coils when it is twisted. We model the cord by a knot, which we define to be an oriented closed curve in three-dimensional space. -

A Remarkable 20-Crossing Tangle Shalom Eliahou, Jean Fromentin
A remarkable 20-crossing tangle Shalom Eliahou, Jean Fromentin To cite this version: Shalom Eliahou, Jean Fromentin. A remarkable 20-crossing tangle. 2016. hal-01382778v2 HAL Id: hal-01382778 https://hal.archives-ouvertes.fr/hal-01382778v2 Preprint submitted on 16 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A REMARKABLE 20-CROSSING TANGLE SHALOM ELIAHOU AND JEAN FROMENTIN Abstract. For any positive integer r, we exhibit a nontrivial knot Kr with r− r (20·2 1 +1) crossings whose Jones polynomial V (Kr) is equal to 1 modulo 2 . Our construction rests on a certain 20-crossing tangle T20 which is undetectable by the Kauffman bracket polynomial pair mod 2. 1. Introduction In [6], M. B. Thistlethwaite gave two 2–component links and one 3–component link which are nontrivial and yet have the same Jones polynomial as the corre- sponding unlink U 2 and U 3, respectively. These were the first known examples of nontrivial links undetectable by the Jones polynomial. Shortly thereafter, it was shown in [2] that, for any integer k ≥ 2, there exist infinitely many nontrivial k–component links whose Jones polynomial is equal to that of the k–component unlink U k. -

Introduction to Vassiliev Knot Invariants First Draft. Comments
Introduction to Vassiliev Knot Invariants First draft. Comments welcome. July 20, 2010 S. Chmutov S. Duzhin J. Mostovoy The Ohio State University, Mansfield Campus, 1680 Univer- sity Drive, Mansfield, OH 44906, USA E-mail address: [email protected] Steklov Institute of Mathematics, St. Petersburg Division, Fontanka 27, St. Petersburg, 191011, Russia E-mail address: [email protected] Departamento de Matematicas,´ CINVESTAV, Apartado Postal 14-740, C.P. 07000 Mexico,´ D.F. Mexico E-mail address: [email protected] Contents Preface 8 Part 1. Fundamentals Chapter 1. Knots and their relatives 15 1.1. Definitions and examples 15 § 1.2. Isotopy 16 § 1.3. Plane knot diagrams 19 § 1.4. Inverses and mirror images 21 § 1.5. Knot tables 23 § 1.6. Algebra of knots 25 § 1.7. Tangles, string links and braids 25 § 1.8. Variations 30 § Exercises 34 Chapter 2. Knot invariants 39 2.1. Definition and first examples 39 § 2.2. Linking number 40 § 2.3. Conway polynomial 43 § 2.4. Jones polynomial 45 § 2.5. Algebra of knot invariants 47 § 2.6. Quantum invariants 47 § 2.7. Two-variable link polynomials 55 § Exercises 62 3 4 Contents Chapter 3. Finite type invariants 69 3.1. Definition of Vassiliev invariants 69 § 3.2. Algebra of Vassiliev invariants 72 § 3.3. Vassiliev invariants of degrees 0, 1 and 2 76 § 3.4. Chord diagrams 78 § 3.5. Invariants of framed knots 80 § 3.6. Classical knot polynomials as Vassiliev invariants 82 § 3.7. Actuality tables 88 § 3.8. Vassiliev invariants of tangles 91 § Exercises 93 Chapter 4. -

Splitting Numbers of Links
Splitting numbers of links Jae Choon Cha, Stefan Friedl, and Mark Powell Department of Mathematics, POSTECH, Pohang 790{784, Republic of Korea, and School of Mathematics, Korea Institute for Advanced Study, Seoul 130{722, Republic of Korea E-mail address: [email protected] Mathematisches Institut, Universit¨atzu K¨oln,50931 K¨oln,Germany E-mail address: [email protected] D´epartement de Math´ematiques,Universit´edu Qu´ebec `aMontr´eal,Montr´eal,QC, Canada E-mail address: [email protected] Abstract. The splitting number of a link is the minimal number of crossing changes between different components required, on any diagram, to convert it to a split link. We introduce new techniques to compute the splitting number, involving covering links and Alexander invariants. As an application, we completely determine the splitting numbers of links with 9 or fewer crossings. Also, with these techniques, we either reprove or improve upon the lower bounds for splitting numbers of links computed by J. Batson and C. Seed using Khovanov homology. 1. Introduction Any link in S3 can be converted to the split union of its component knots by a sequence of crossing changes between different components. Following J. Batson and C. Seed [BS13], we define the splitting number of a link L, denoted by sp(L), as the minimal number of crossing changes in such a sequence. We present two new techniques for obtaining lower bounds for the splitting number. The first approach uses covering links, and the second method arises from the multivariable Alexander polynomial of a link. Our general covering link theorem is stated as Theorem 3.2. -

Comultiplication in Link Floer Homologyand Transversely Nonsimple Links
Algebraic & Geometric Topology 10 (2010) 1417–1436 1417 Comultiplication in link Floer homology and transversely nonsimple links JOHN ABALDWIN For a word w in the braid group Bn , we denote by Tw the corresponding transverse 3 braid in .R ; rot/. We exhibit, for any two g; h Bn , a “comultiplication” map on 2 link Floer homology ˆ HFL.m.Thg// HFL.m.Tg#Th// which sends .Thg/ z W ! z to .z Tg#Th/. We use this comultiplication map to generate infinitely many new examples of prime topological link types which are not transversely simple. 57M27, 57R17 e e 1 Introduction Transverse links feature prominently in the study of contact 3–manifolds. They arise very naturally – for instance, as binding components of open book decompositions – and can be used to discern important properties of the contact structures in which they sit (see Baker, Etnyre and Van Horn-Morris [1, Theorem 1.15] for a recent example). Yet, 3 transverse links, even in the standard tight contact structure std on R , are notoriously difficult to classify up to transverse isotopy. A transverse link T comes equipped with two “classical” invariants which are preserved under transverse isotopy: its topological link type and its self-linking number sl.T /. For transverse links with more than one component, it makes sense to refine the notion of self-linking number as follows. Let T and T 0 be two transverse representatives of some l –component topological link type, and suppose there are labelings T T1 T D [ [ l and T T T of the components of T and T such that 0 D 10 [ [ l0 0 (1) there is a topological isotopy sending T to T 0 which sends Ti to Ti0 for each i , (2) sl.S/ sl.S / for any sublinks S Tn Tn and S T T . -

Lecture 6 Braids Braids, Like Knots and Links, Are Curves in R3 with A
Lecture 6 Braids Braids, like knots and links, are curves in R3 with a natural composition operation. They have the advantage of forming a group under the composition operation (unlike knots, inverse braids always exist). This group is denoted by Bn. The subscript n is a natural number, so that there is an infinite series of braid groups B1 ⊂ B2 ⊂ · · · ⊂ Bn ⊂ ::: At first we shall define and study these groups geometrically, then look at them as a purely algebraic object via their presenta- tion obtained by Emil Artin (who actually invented braids in the 1920ies). Then we shall see that braids are related to knots and links via a geometric modification called closure and study the consequences of this relationship. We note at once that braids play an important role in many fields of mathematics and theoretical physics, exemplified by such important terms as “braid cohomology”, and this is not due to their relationship to knots. But here we are interested in braids mainly because of this relationship. 6.1. Geometric braids A braid in n strands is a set consisting of n pairwise noninter- secting polygonal curves (called strands) in R3 joining n points aligned on a horizontal line L to n points with the same x; y coordinates aligned on a horizontal line L0 parallel to L and located lower than L; the strands satisfy the following condition: when we move downward along any strand from a point on L to 1 2 a point on L0, the tangent vectors of this motion cannot point upward (i.e., the z-coordinate of these vectors is always negative). -

Knot Theory and the Alexander Polynomial
Knot Theory and the Alexander Polynomial Reagin Taylor McNeill Submitted to the Department of Mathematics of Smith College in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors Elizabeth Denne, Faculty Advisor April 15, 2008 i Acknowledgments First and foremost I would like to thank Elizabeth Denne for her guidance through this project. Her endless help and high expectations brought this project to where it stands. I would Like to thank David Cohen for his support thoughout this project and through- out my mathematical career. His humor, skepticism and advice is surely worth the $.25 fee. I would also like to thank my professors, peers, housemates, and friends, particularly Kelsey Hattam and Katy Gerecht, for supporting me throughout the year, and especially for tolerating my temporary insanity during the final weeks of writing. Contents 1 Introduction 1 2 Defining Knots and Links 3 2.1 KnotDiagramsandKnotEquivalence . ... 3 2.2 Links, Orientation, and Connected Sum . ..... 8 3 Seifert Surfaces and Knot Genus 12 3.1 SeifertSurfaces ................................. 12 3.2 Surgery ...................................... 14 3.3 Knot Genus and Factorization . 16 3.4 Linkingnumber.................................. 17 3.5 Homology ..................................... 19 3.6 TheSeifertMatrix ................................ 21 3.7 TheAlexanderPolynomial. 27 4 Resolving Trees 31 4.1 Resolving Trees and the Conway Polynomial . ..... 31 4.2 TheAlexanderPolynomial. 34 5 Algebraic and Topological Tools 36 5.1 FreeGroupsandQuotients . 36 5.2 TheFundamentalGroup. .. .. .. .. .. .. .. .. 40 ii iii 6 Knot Groups 49 6.1 TwoPresentations ................................ 49 6.2 The Fundamental Group of the Knot Complement . 54 7 The Fox Calculus and Alexander Ideals 59 7.1 TheFreeCalculus ............................... -

How Can We Say 2 Knots Are Not the Same?
How can we say 2 knots are not the same? SHRUTHI SRIDHAR What’s a knot? A knot is a smooth embedding of the circle S1 in IR3. A link is a smooth embedding of the disjoint union of more than one circle Intuitively, it’s a string knotted up with ends joined up. We represent it on a plane using curves and ‘crossings’. The unknot A ‘figure-8’ knot A ‘wild’ knot (not a knot for us) Hopf Link Two knots or links are the same if they have an ambient isotopy between them. Representing a knot Knots are represented on the plane with strands and crossings where 2 strands cross. We call this picture a knot diagram. Knots can have more than one representation. Reidemeister moves Operations on knot diagrams that don’t change the knot or link Reidemeister moves Theorem: (Reidemeister 1926) Two knot diagrams are of the same knot if and only if one can be obtained from the other through a series of Reidemeister moves. Crossing Number The minimum number of crossings required to represent a knot or link is called its crossing number. Knots arranged by crossing number: Knot Invariants A knot/link invariant is a property of a knot/link that is independent of representation. Trivial Examples: • Crossing number • Knot Representations / ~ where 2 representations are equivalent via Reidemester moves Tricolorability We say a knot is tricolorable if the strands in any projection can be colored with 3 colors such that every crossing has 1 or 3 colors and or the coloring uses more than one color.