A Simple Key to Canal Water Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Beginner's Guide to Boating on Inland Waterways
Ti r A Beginner’s Guide To Boating On Inland Waterways Take to the water with British Waterways and the National Rivers Authority With well over 4,000 km (2,500 miles) of rivers and canals to explore, from the south west of England up to Scotland, our inland waterways offer plenty of variety for both the casual boater and the dedicated enthusiast. If you have ever experienced the pleasures of 'messing about on boats', you will know what a wealth of scenery and heritage inland waterways open up to us, and the unique perspective they provide. Boating is fun and easy. This pack is designed to help you get afloat if you are thinking about buying a boat. Amongst other useful information, it includes details of: Navigation Authorities British Waterways (BW) and the National Rivers Authority (NRA), which is to become part of the new Environment Agency for England and Wales on 1 April 1996, manage most of our navigable rivers and canals. We are responsible for maintaining the waterways and locks, providing services for boaters and we licence and manage boats. There are more than 20 smaller navigation authorities across the country. We have included information on some of these smaller organisations. Licences and Moorings We tell you everything you need to know from, how to apply for a licence to how to find a permanent mooring or simply a place for «* ^ V.’j provide some useful hints on buying a boat, includi r, ...V; 'r 1 builders, loans, insurance and the Boat Safety Sch:: EKVIRONMENT AGENCY Useful addresses A detailed list of useful organisations and contacts :: : n a t io n a l libra ry'& ■ suggested some books we think will help you get t information service Happy boating! s o u t h e r n r e g i o n Guildbourne House, Chatsworth Road, W orthing, West Sussex BN 11 1LD ENVIRONMENT AGENCY 1 Owning a Boat Buying a Boat With such a vast.range of boats available to suit every price range, . -

Download Brochure
WELCOME to BROADOAKS PAR K — Inspirational homes for An exclusive development of luxurious Built by Ernest Seth-Smith, the striking aspirational lifestyles homes by award winning housebuilders Broadoaks Manor will create the Octagon Developments, Broadoaks Park centrepiece of Broadoaks Park. offers the best of countryside living in Descending from a long-distinguished the heart of West Byfleet, coupled with line of Scottish architects responsible for excellent connections into London. building large areas of Belgravia, from Spread across 25 acres, the gated parkland Eaton Square to Wilton Crescent, Seth-Smith estate offers a mixture of stunning homes designed the mansion and grounds as the ranging from new build 2 bedroom ultimate country retreat. The surrounding apartments and 3 - 6 bedroom houses, lodges and summer houses were added to beautifully restored and converted later over the following 40 years, adding apartments and a mansion house. further gravitas and character to the site. Surrey LIVING at its BEST — Painshill Park, Cobham 18th-century landscaped garden with follies, grottoes, waterwheel and vineyard, plus tearoom. Experience the best of Surrey living at Providing all the necessities, a Waitrose Retail therapy Broadoaks Park, with an excellent range of is located in the village centre, and Guildford’s cobbled High Street is brimming with department stores restaurants, parks and shopping experiences for a wider selection of shops, Woking and and independent boutiques alike, on your doorstep. Guildford town centres are a short drive away. offering one of the best shopping experiences in Surrey. Home to artisan bakeries, fine dining restaurants Opportunities to explore the outdoors are and cosy pubs, West Byfleet offers plenty plentiful, with the idyllic waterways of the of dining with options for all occasions. -
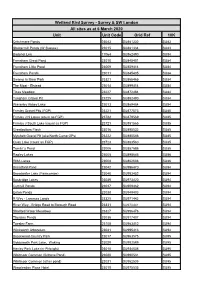
Unit Unit Code Grid Ref 10K Wetland Bird Survey
Wetland Bird Survey - Surrey & SW London All sites as at 6 March 2020 Unit Unit Code Grid Ref 10K Critchmere Ponds 23043 SU881332 SU83 Shottermill Ponds (W Sussex) 23015 SU881334 SU83 Badshot Lea 17064 SU862490 SU84 Frensham Great Pond 23010 SU845401 SU84 Frensham Little Pond 23009 SU859414 SU84 Frensham Ponds 23011 SU845405 SU84 Swamp in Moor Park 23321 SU865465 SU84 The Moat - Elstead 23014 SU899414 SU84 Tices Meadow 23227 SU872484 SU84 Tongham Gravel Pit 23225 SU882490 SU84 Waverley Abbey Lake 23013 SU869454 SU84 Frimley Gravel Pits (FGP) 23221 SU877573 SU85 Frimley J N Lakes (count as FGP) 23722 SU879569 SU85 Frimley J South Lake (count as FGP) 23721 SU881565 SU85 Greatbottom Flash 23016 SU895532 SU85 Mytchett Gravel Pit (aka North Camp GPs) 23222 SU885546 SU85 Quay Lake (count as FGP) 23723 SU883560 SU85 Tomlin`s Pond 23006 SU887586 SU85 Rapley Lakes 23005 SU898646 SU86 RMA Lakes 23008 SU862606 SU86 Broadford Pond 23042 SU996470 SU94 Broadwater Lake (Farncombe) 23040 SU983452 SU94 Busbridge Lakes 23039 SU973420 SU94 Cuttmill Ponds 23037 SU909462 SU94 Enton Ponds 23038 SU949403 SU94 R Wey - Lammas Lands 23325 SU971442 SU94 River Wey - Bridge Road to Borough Road 23331 SU970441 SU94 Shalford Water Meadows 23327 SU996476 SU94 Thursley Ponds 23036 SU917407 SU94 Tuesley Farm 23108 SU963412 SU94 Winkworth Arboretum 23041 SU995413 SU94 Brookwood Country Park 23017 SU963575 SU95 Goldsworth Park Lake, Woking 23029 SU982589 SU95 Henley Park Lake (nr Pirbright) 23018 SU934536 SU95 Whitmoor Common (Brittons Pond) 23020 SU990531 SU95 Whitmoor -

LONDON METROPOLITAN ARCHIVES Page 1 BRITISH WATERWAYS BOARD
LONDON METROPOLITAN ARCHIVES Page 1 BRITISH WATERWAYS BOARD ACC/2423 Reference Description Dates LEE CONSERVANCY BOARD ENGINEER'S OFFICE Engineers' reports and letter books LEE CONSERVANCY BOARD: ENGINEER'S REPORTS ACC/2423/001 Reports on navigation - signed copies 1881 Jan-1883 Lea navigation Dec 1 volume ACC/2423/002 Reports on navigation - signed copies 1884 Jan-1886 Lea navigation Dec 1 volume ACC/2423/003 Reports on navigation - signed copies 1887 Jan-1889 Lea navigation Dec 1 volume ACC/2423/004 Reports on navigation - signed copies 1890 Jan-1893 Lea navigation Dec 1 volume ACC/2423/005 Reports on navigation - signed copies 1894 Jan-1896 Lea navigation Dec 1 volume ACC/2423/006 Reports on navigation - signed copies 1897 Jan-1899 Lea navigation Dec 1 volume ACC/2423/007 Reports on navigation - signed copies 1903 Jan-1903 Lea navigation Dec 1 volume ACC/2423/008 Reports on navigation - signed copies 1904 Jan-1904 Lea navigation Dec 1 volume ACC/2423/009 Reports on navigation - signed copies 1905 Jan-1905 Lea navigation Dec 1 volume ACC/2423/010 Reports on navigation - signed copies 1906 Jan-1906 Lea navigation Dec 1 volume LONDON METROPOLITAN ARCHIVES Page 2 BRITISH WATERWAYS BOARD ACC/2423 Reference Description Dates ACC/2423/011 Reports on navigation - signed copies 1908 Jan-1908 Lea navigation/ stort navigation Dec 1 volume ACC/2423/012 Reports on navigation - signed copies 1912 Jan-1912 Lea navigation/ stort navigation Dec 1 volume ACC/2423/013 Reports on navigation - signed copies 1913 Jan-1913 Lea navigation/ stort navigation -

Premises, Sites Etc Within 30 Miles of Harrington Museum Used for Military Purposes in the 20Th Century
Premises, Sites etc within 30 miles of Harrington Museum used for Military Purposes in the 20th Century The following listing attempts to identify those premises and sites that were used for military purposes during the 20th Century. The listing is very much a works in progress document so if you are aware of any other sites or premises within 30 miles of Harrington, Northamptonshire, then we would very much appreciate receiving details of them. Similarly if you spot any errors, or have further information on those premises/sites that are listed then we would be pleased to hear from you. Please use the reporting sheets at the end of this document and send or email to the Carpetbagger Aviation Museum, Sunnyvale Farm, Harrington, Northampton, NN6 9PF, [email protected] We hope that you find this document of interest. Village/ Town Name of Location / Address Distance to Period used Use Premises Museum Abthorpe SP 646 464 34.8 km World War 2 ANTI AIRCRAFT SEARCHLIGHT BATTERY Northamptonshire The site of a World War II searchlight battery. The site is known to have had a generator and Nissen huts. It was probably constructed between 1939 and 1945 but the site had been destroyed by the time of the Defence of Britain survey. Ailsworth Manor House Cambridgeshire World War 2 HOME GUARD STORE A Company of the 2nd (Peterborough) Battalion Northamptonshire Home Guard used two rooms and a cellar for a company store at the Manor House at Ailsworth Alconbury RAF Alconbury TL 211 767 44.3 km 1938 - 1995 AIRFIELD Huntingdonshire It was previously named 'RAF Abbots Ripton' from 1938 to 9 September 1942 while under RAF Bomber Command control. -

British Waterways Board General Canal Bye-Laws
BRITISH WATERWAYS BOARD GENERAL CANAL BYE-LAWS 1965 BRITISH WATERWAYS BOARD BYE-LAWS ____________________ for regulation of the canals belonging to or under the control of the British Waterways Board (other than the canals specified in Bye-law 1) made pursuant to the powers of the British Transport Commission Act, 1954. (N.B. – The sub-headings and marginal notes do not form part of these Bye-laws). Application of Bye-laws Application of 1. These Bye-laws shall apply to every canal or inland navigation in Bye-Laws England and Wales belonging to or under the control of the British Waterways Board except the following canals: - (a) The Lee and Stort Navigation (b) the Gloucester and Sharpness Canal (c) the River Severn Navigation which are more particularly defined in the Schedule hereto. Provided that where the provisions of any of these Bye-laws are limited by such Bye-law to any particular canal or locality then such Bye-law shall apply only to such canal or locality to which it is so limited. These Bye-laws shall come into operation at the expiration of twenty-eight days after their confirmation by the Minister of Transport as from which date all existing Bye-laws applicable to the canals and inland navigations to which these Bye-laws apply (other than those made under the Explosives Act 1875, and the Petroleum (Consolidation) Act 1928) shall cease to have effect, without prejudice to the validity of anything done thereunder or to any liability incurred in respect of any act or omission before the date of coming into operation of these Bye-laws. -

Coarse Fishing Close Season on English Rivers
Coarse fishing close season on English rivers Appendix 1 – Current coarse fish close season arrangements The close season on different waters In England, there is a coarse fish close season on all rivers, some canals and some stillwaters. This has not always been the case. In the 1990s, only around 60% of the canal network had a close season and in some regions, the close season had been dispensed with on all stillwaters. Stillwaters In 1995, following consultation, government confirmed a national byelaw which retained the coarse fish close season on rivers, streams, drains and canals, but dispensed with it on most stillwaters. The rationale was twofold: • Most stillwaters are discrete waterbodies in single ownership. Fishery owners can apply bespoke angling restrictions to protect their stocks, including non-statutory close times. • The close season had been dispensed with on many stillwaters prior to 1995 without apparent detriment to those fisheries. This presented strong evidence in favour of removing it. The close season is retained on some Sites of Special Scientific Interest (SSSIs) and the Norfolk and Suffolk Broads, as a precaution against possible damage to sensitive wildlife - see Appendix 1. This consultation is not seeking views on whether the close season should be retained on these stillwaters While most stillwater fishery managers have not re-imposed their own close season rules, some have, either adopting the same dates as apply to rivers or tailoring them to their waters' specific needs. Canals The Environment Agency commissioned a research project in 1997 to examine the evidence around the close season on canals to identify whether or not angling during the close season was detrimental to canal fisheries. -

Questionnaire Results ‘Shaping the Future of West Byfleet’ November 2014 Questionnaire 1
Questionnaire Results ‘Shaping the Future of West Byfleet’ November 2014 Questionnaire 1. Do you think more housing is needed in West Byfleet ? ? Yes 33% Y No 55% Don’t Know 12% 33N 5512 2. If yes which of the following do you think we need more of ? 3. Do you want land to be set aside for Travellers / Romany in West Byfleet ? ? Y Yes 2% No 94% Don’t Know 4% N 4. If more homes were to be built in West Byfleet changes/ improvements to the infrastructure are likely to be needed. 2 Please indicate your level of feeling 944 5. Do you think all new housing developments should be required to provide adequate off street parking? N ? Yes 96% No 3% Don’t Know 1% Y 6. Do you think flats should only be permitted to be built in the 96 commerical centre of West Byfleet & on31 the A245 ? ? Yes 64% No 22% 64N Y 2214 Don’t Know 14% 7. How important to you is it that the style & character of housing in your street is retained ? Not Important 3% Neutral 12% Important 28% Very Important Important Very Important 57% 8. When considering new developments, do you think an overall high standard of design and use of quality materials is more or less important than matching the style of 3 existing housing on a street ? 1228+57 ? More 60% Less 26% 60Less More 2614 Don’t Know 14% 9. Do you think back garden developments should be allowed in West Byfleet ? Y Yes 12% M No 61% Maybe 27% N 1210. -

Beating the Bounds
BEATING THE BOUNDS Iain Wakeford © 2014 ou might be forgiven, if you have looked at the previous pages on this site, in thinking that only Woking and Chertsey were recorded in this area before the writing of the Domesday Book. But Byfleet was recorded in 727 - fleot or fleet being an old Y name for a stream (its location being ‘by [the] fleet’) - with Pyrford following in 956 and Send in about 960-2. Crockford may refer to ‘crocks’ of pottery, being deposited near the ford as some offering to the ancient river gods! Nearby the ‘herestreat’ (a military road), may mark the route of a Roman Road (mentioned in my article on the Roman period). orsell isn’t recorded until the 13th The 7th century bounds of Chertsey Abbey The later 9th century bounds of Chertsey, century, but a number of other ‘minor’ record many local places for the first time recorded in King Alfred’s Charter of about 889, H place names do make their way into including Weybridge, Woburn and Crockford at are slightly different and mention for the first the records before the Norman Conquest, Addlestone, as well as Mimbridge (bridge near time Wintersells (Wyntredeshulle), Fullbrook including Egley (or Egceanlea – Ecga’s Clearing) the field of mint) and Sythwood (possibly (fule brok – or foul brook) and Durnford – as in the road between Woking & Mayford, ‘scythe shaped wood’) at Horsell. (derneforde) – the latter apparently meaning a recorded in about 1005 (if the 12th century ‘secret’ or hidden ford. records of Eynsham Abbey in Oxfordshire are to Of course the present day Sythwood on Goldsworth Park is just a modern Wintersells was at one time a farm off Oyster be believed). -

South1vimlands Arc'haeology
SOUTH1VIMLANDS ARC'HAEOLOGY The Newsletter of the Council for British Archaeology, South Midlands Group (Bedfordshire, Buckinghamshire, Northamptonshire, Oxfordshire) NUIVIBER 33, 2003 CONTENTS Page Editorial Bedfordshire 1 Buckinghamshire 21 Northamptonshire 37 Oxfordshire 57 Index 113 Notes for Contributors 125 It should be noted that the reports in this volume refer, in the main, to work carried out in 2002. EDITOR: Barry Home CHAIRMAN: Ted Legg 'Beaumont' 17 Napier Street Church End Bletchley Edlesborough Milton Keynes Dunstable, Beds MK2 2NF LU6 2EP HON SEC: Vacant TREASURER: Gerry Mico 6 Rowan Close Brackley NN13 6PB Typeset by Barry Home ISSN 0960-7552 EDITORIAL Welcome to volume 33. The cumulative index to volumes 1-33, is available on the website at WWW.britarch.ac.uk/smaindex If anyone wishes to have a copy for their own PC would they please send me a 3.5" disk and a stamped addressed envelope and I will provide them with a copy. A number of new organisations have provided reports and this is very encouraging. However, some organisations continue to provide no report of their work in the area, in particular I know of one which has done work in it on a gas pipeline and a churchyard near where I live. I'm sure there are others. County archaeologists and peers must apply pressure to these defaulters. Through this editorial could I please request that when contract archaeologists do work in an area they make their presence known to the local archaeological society, because it is that society to which the public will address questions about what is going on; it does help archaeology's image if we all seem to be working together. -

Map of London's Waterways
LONDON WATERWAYS Bishop’s Stortford A1250 BBISHOP’SISHOP’S STORTFORDSTORTFORD WareWare SouthSouth MillMill LockLock LockLock M11 HertfordHertford LockLock TwyfordTwyford Ware LockLock GreatGreat Hertford A1184 A119 A1060 WWAREARE SpellbrookSpellbrook HallingburyHallingbury HHERTFORDERTFORD EEASTAST LockLock LittleLittle A414 A1170 HardmeadHardmead LockLock HallingburyHallingbury TednamburyTednambury KEY StansteadStanstead LockLock LockLock Managed by British Waterways SSTT MMARGARETSARGARETS SawbridgeworthSawbridgeworth Stanstead SawbridgeworthSawbridgeworth LockLock Canal continues Abbotts RiverRiver SStorttort NavigationNavigation SSAWBRIDGEWORTHAWBRIDGEWORTH Tunnel RROYDONOYDON HarlowHarlow SheeringSheering MillMill LockLock HunsdonHunsdon Eastwick LockLock Other Waterways MillMill LockLock BurntBurnt MillMill FeakesFeakes LockLock WareWare RRYEYE HHOUSEOUSE BrickBrick LockLock LockLock Lock and Lock Name LockLock Feilde’sFeilde’s ParndonParndon HHARLOWARLOW MMILLILL RoydonRoydon LattonLatton LockLock HoddesdonHoddesdon WeirWeir LockLock MillMill LockLock A1169 Parkland LockLock LowerLower LockLock HHARLOWARLOW TTOWNOWN Wetland Harlow A1019 M11 BBROXBOURNEROXBOURNE British Waterways Main Office Dobb’sDobb’s WeirWeir LockLock A414 DOCKLANDS British Waterways Local Office CarthagenaCarthagena LockLock A10 M11 Motorway A10 Main roads NNazeingazeing MarshesMarshes National Rail Station AqueductAqueduct LockLock London Underground Station CheshuntCheshunt LockLock Docklands Light Railway Station CCHESHUNTHESHUNT WalthamWaltham CommonCommon -

Office Investment with Development Potential
OFFICE INVESTMENT WITH DEVELOPMENT POTENTIAL GENERAL ELECTRIC, BOUGHTON ROAD, RUGBY CV21 1BU OFFICE INVESTMENT WITH DEVELOPMENT POTENTIAL GENERAL ELECTRIC, BOUGHTON ROAD, RUGBY CV21 1BU Investment Summary Opportunity to acquire a single let HQ office building with Let to GE Energy Power Conversion UK Limited, reversionary development potential a subsidiary of General Electric 3 storey office totalling86,983 sq ft sitting on a large site of 6.17 acres Potential redevelopment for residential on expiry of the lease (STP) Low site coverage of 19% Current rent of £462,000 per annum (just £5.31 psf) Excellent location 2 miles north of Rugby town centre in Offers in excess of £4,000,000 (STC) Warwickshire, off junction 1 of the M6 Net Initial Yield of 10.84% Let for a term of 5 years commencing 1st January 2018, expiring Low capital value of just £46 psf 31st December 2022 (1.93 years unexpired), and contracted out of £648,000 per acre the L&T Act 1954 OFFICE INVESTMENT WITH DEVELOPMENT POTENTIAL GENERAL ELECTRIC, BOUGHTON ROAD, RUGBY CV21 1BU Electrical Boughton Road Substation Rugby CV21 1BU TO COVENTRY TO M1 M6 M6 J1 M6 RUGBY GATEWAY CENTRAL PARK SWIFT VALLEY INDUSTRIAL ESTATE A426 GLEBE FARM INDUSTRIAL ESTATE Boughton Rd A426 CALDECOTT MANOR GE MANUFACTURING FACILITY TO RUGBY TOWN CENTRE OFFICE INVESTMENT WITH DEVELOPMENT POTENTIAL GENERAL ELECTRIC, BOUGHTON ROAD, RUGBY CV21 1BU RUGBY TOWN CENTRE GE MANUFACTURING FACILITY RUGBY Electrical Substation A426 A426 TO M6 TO RUGBY GATEWAY CALDECOTT MANOR CALDECOTT MANOR TO SWIFT VALLEY INDUSTRIAL