(12) Patent Application Publication (10) Pub. No.: US 2011/0062721 A1 Sirdeshpande Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methanation of CO2 - Storage of Renewable Energy in a Gas Distribution System Tanja Schaaf*, Jochen Grünig, Markus Roman Schuster, Tobias Rothenfluh and Andreas Orth
Schaaf et al. Energy, Sustainability and Society ( DOI 10.1186/s13705-014-0029-1 REVIEW Open Access Methanation of CO2 - storage of renewable energy in a gas distribution system Tanja Schaaf*, Jochen Grünig, Markus Roman Schuster, Tobias Rothenfluh and Andreas Orth Abstract This article presents some crucial findings of the joint research project entitled «Storage of electric energy from renewable sources in the natural gas grid-water electrolysis and synthesis of gas components». The project was funded by BMBF and aimed at developing viable concepts for the storage of excess electrical energy from wind and solar power plants. The concept presented in this article suggests the conversion of CO2-containing gases into methane in a pressurized reactor using hydrogen produced via electrolysis. The produced gas can be upgraded to synthetic natural gas (SNG) and fed into the well-developed German natural gas grid. This concept benefits from the high storage capacity of the German gas grid and does not require any extensions of the current gas or power grid. The reaction heat released by the exothermic methanation reaction leads to a temperature rise of the gas in the fixed bed catalyst of the reactor. The conversion of carbon dioxide is limited in accordance to the chemical equilibrium which depends strongly on temperature and pressure. For maximum carbon dioxide conversion, it is convenient to split the methanation into several stages adding cooling sections in between. This article focuses on the methanation process and its transfer onto an industrial scale evaluating the different plant capacities and feedstock mixtures used. The methanation takes place in a staged fixed bed reactor. -

CHE 670 Outline
CHE 670 - Sustainability Seminar Kansas State University Sustainability Seminar – Summer 2017……………………………………….…...1 Sustainability Seminar – Summer 2016……………………………………………2 Sustainability Seminar – Summer 2015……………………………………………3 Sustainability Seminar – Summer 2014……………………………………………4 Sustainability Seminar – January 2014…………………………………………….5 Sustainability Seminar – Summer 2013……………………………………………6 Sustainability Seminar – Summer 2012……………………………………………7 Sustainability Seminar – January 2012………………………………………….…8 Sustainability Seminar – Summer 2011……………………………………………9 Sustainability Seminar – Summer 2010…………………………………………..10 Sustainability Seminar – January 2010 ………………………………………….11 Sustainability Seminar – Summer 2009………………………………………....12 Sustainability Seminar – Fall 2008 ………………………………………….....13 Sustainability Seminar – January 2008……………………………………….….14 0 Agenda CHE 670 Sustainability Seminar Summer 2017 Fridays at 8:30 a.m. June 2 - John Harrington - Global Change June 9 - Lawrence Davis - Land Sustainability and Reclamation June 16 – Warren White – Wind Energy Basics June 23 – Keith Hohn – Catalysis for Renewable Energy June 30 – Vahid Rahmani – Water Sustainability July 7 – Oral Saulters – Sustainability Competencies and Sustainable Development Policy July 14 – Hongyu Wu – Smart Electrical Power Grid with Renewable Energy July 21 - Trisha Moore – Ecological Engineering and Sustainable Ecosystems July 22 - Dialog on Sustainability at K-State – All Day July 28 – Bimal Paul – Sustainable Development August 5 - Student Oral Presentations * The July 22 -

Conversion of Co2 to Syngas and Synthetic Natural Gas (Sng): Technologies and Markets
THE CATALYST GROUP RESOURCES™ CONVERSION OF CO2 TO SYNGAS AND SYNTHETIC NATURAL GAS (SNG): TECHNOLOGIES AND MARKETS A techno-economic investigation commissioned by the members of the Carbon Dioxide Capture & Conversion (CO2CC) Program Client Private December 2013 Gwynedd Office Park ● P.O. Box 680 ● Spring House, PA 19477 ● Phone: 215-628-4447 ● Fax: 215-628-2267 E-mail: [email protected] ● Web Site: www.catalystgrp.com The Carbon Dioxide Capture & Conversion (CO2CC) Program The CO2CC Program is a membership-directed consortium whose members are involved in the development, monitoring and utilization of the “state-of-the-art” in technological progress and commercial implementation of carbon dioxide capture/clean-up and conversion. By the direction of the member companies (through balloting and other interactive means), the program delivers a range of timely and insightful information and analyses which are accessible exclusively to members and protected by confidentiality agreements. The objective is to document technically and commercially viable options for CO2 capture/clean-up as well as its conversion into useful products which meaningfully address the challenges posed by CO2 life-cycle and overall sustainability issues. Members receive three in-depth CO2CC Techno-economic Reports which are written by leading scientists and experienced industry professionals in areas selected by the membership (via ballot); weekly CO2CC Communiqués (delivered via e-mail) which provide the latest updates on technical breakthroughs, commercial events and exclusive development opportunities; and attendance at the CO2CC Program Annual Meeting. The Carbon Dioxide Capture & Conversion (CO2CC) Program is available on a membership basis from The Catalyst Group Resources (TCGR). For further details, please contact John J. -

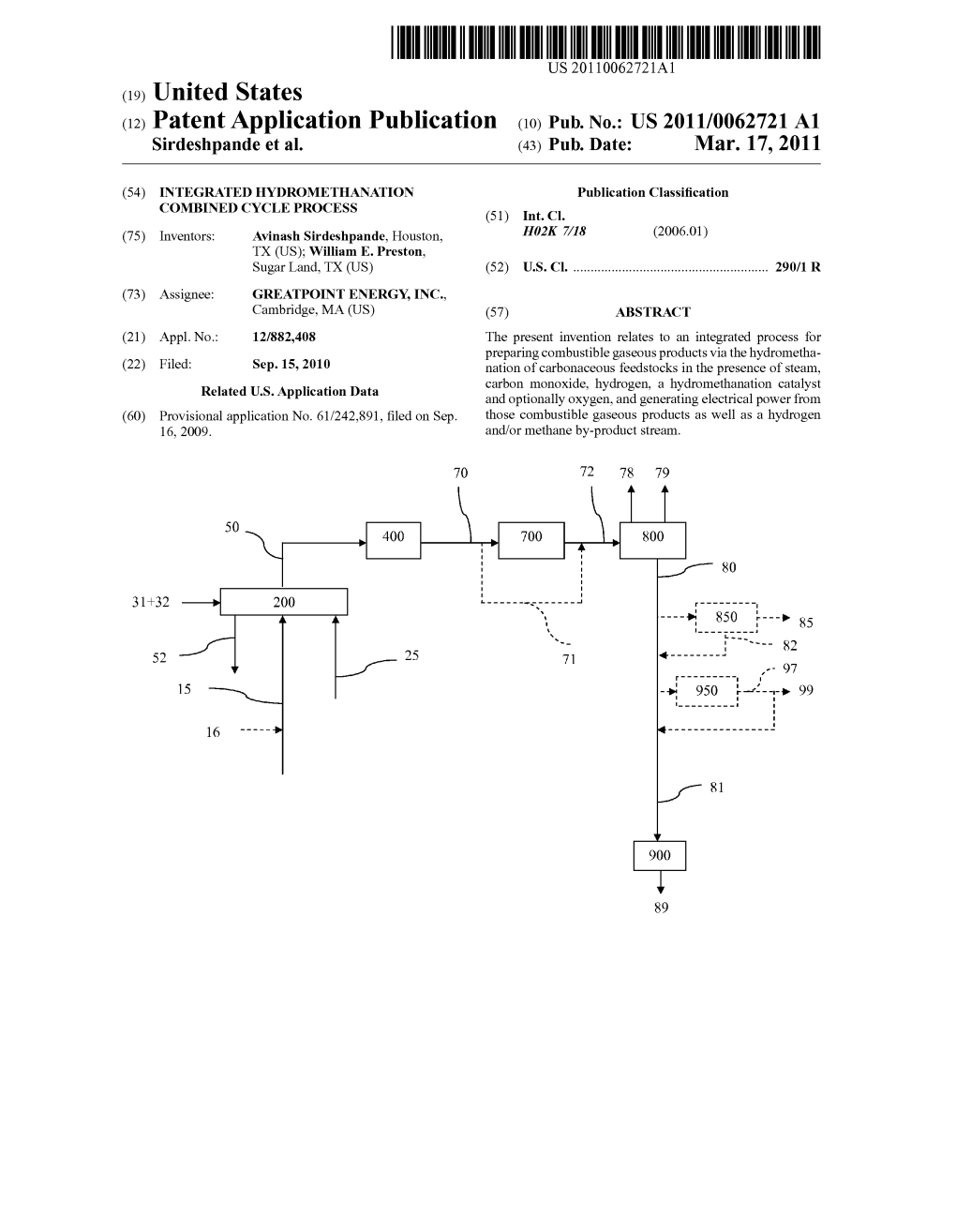
(12) United States Patent (10) Patent No.: US 9,034,061 B2 Robinson Et Al
USOO9034061 B2 (12) United States Patent (10) Patent No.: US 9,034,061 B2 Robinson et al. (45) Date of Patent: *May 19, 2015 (54) AGGLOMERATED PARTICULATE CI0J 3/72 (2013.01); F23K I/00 (2013.01); LOW-RANK COAL FEEDSTOCKAND USES CIOL 5/10 (2013.01); CIOL 5/363 (2013.01) THEREOF (58) Field of Classification Search CPC .............. C10L 5/04; C10J 3/72: F23L 7/007; (71) Applicant: GreatPoint Energy, Inc., Cambridge, F23K1 f(00 MA (US) USPC ............................ 44/550,592, 593, 595,596 See application file for complete search history. (72) Inventors: Earl T. Robinson, Lakeland, FL (US); Kenneth P. Keckler, Naperville, IL (56) References Cited (US); Pattabhi K. Raman, Kildeer, IL (US); Avinash Sirdeshpande, Chicago, U.S. PATENT DOCUMENTS IL (US) 2,605.215 A 7/1952 Coghlan (73) Assignee: GreatPoint Energy, Inc., Cambridge, 2,694,923 A 1 1/1954 Welty, Jr. et al. MA (US) (Continued) (*) Notice: Subject to any disclaimer, the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 CA 966660 4, 1975 U.S.C. 154(b) by 0 days. CA 1003217 1, 1977 This patent is Subject to a terminal dis (Continued) claimer. OTHER PUBLICATIONS (21) Appl. No.: 14/039,321 U.S. Appl. No. 12/778,538, filed May 12, 2010, Robinson, et al. (22) Filed: Sep. 27, 2013 (Continued) (65) Prior Publication Data Primary Examiner — Cephia DToomer US 2014/009 1258A1 Apr. 3, 2014 (74) Attorney, Agent, or Firm — McDonnell Boehnen Hulbert & Berghoff LLP Related U.S. Application Data (57) ABSTRACT (60) Provisional application No. -

Next Generation Coal Gasification Technology
Next generation coal gasification technology Ian Barnes CCC/187 ISBN 978-92-9029-507-5 September 2011 copyright © IEA Clean Coal Centre Abstract Worldwide, a small number of integrated gasification combined cycle power plants (IGCC), based on high-efficiency coal gasification technologies, are operated commercially or semi-commercially, a few more are under construction, and a number of demonstration projects, some including carbon capture and sequestration (CCS), are at an advanced stage of planning. Various coal gasification technologies are embodied in these plants including different coal feed systems (dry or slurry), fireproof interiors walls (fire brick or water-cooled tubes), oxidants (oxygen or air), and other factors. Many of these designs are now several decades old but new cycles and systems are emerging to further improve the efficiency of the coal gasification process. This report draws upon the published literature and commentary from experts in industry and academia working in the coal gasification sector to present and summarise recent developments. Acknowledgements The author is grateful for useful discussions and advice freely given by industry and academic professionals. In particular, the contributions of the following are acknowledged: Dr Kourosh E Zanganeh CANMET Dr Manabu Hirata NEDO Mr John Davison IEAGHG Dr John Griffiths Jacobs Ms Jenny Tennant NETL Mr David Butler Canadian Clean Power Coalition Mr Ronald L Schoff EPRI Acronyms and abbreviations AC air compressor ADT acid gas dewpoint temperature AHAT humid air -

A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis
nanomaterials Review A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis Ju Li 1, Cunhua Ma 1,*, Shengjie Zhu 1, Feng Yu 1 , Bin Dai 1 and Dezheng Yang 2,3 1 Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, School of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832003, China; [email protected] (J.L.); [email protected] (S.Z.); [email protected] (F.Y.); [email protected] (B.D.) 2 Laboratory of Plasma Physical Chemistry, School of Physics, Dalian University of Technology, Dalian 116024, China; [email protected] 3 Key Laboratory of Ecophysics, College of Sciences, Shihezi University, Shihezi 832003, China * Correspondence: [email protected]; Tel.: +86-0993-205-8775 Received: 28 August 2019; Accepted: 21 September 2019; Published: 9 October 2019 Abstract: Dielectric barrier discharge plasma is one of the most popular methods to generate nanthermal plasma, which is made up of a host of high-energy electrons, free radicals, chemically active ions and excited species, so it has the property of being prone to chemical reactions. Due to these unique advantages, the plasma technology has been widely used in the catalytic fields. Compared with the conventional method, the heterogeneous catalyst prepared by plasma technology has good dispersion and smaller particle size, and its catalytic activity, selectivity and stability are significantly improved. In addition, the interaction between plasma and catalyst can achieve synergistic effects, so the catalytic effect is further improved. The review mainly introduces the characteristics of dielectric barrier discharge plasma, development trend and its recent advances in catalysis; then, we sum up the advantages of using plasma technology to prepare catalysts. -

Gt2016-57857
Proceedings of ASME Turbo Expo 2016: Turbomachinery Technical Conference and Exposition GT2016 June 13 – 17, 2016, Seoul, South Korea GT2016-57857 Review of Coal-To-Synthetic Natural Gas (SNG) Production Methods and Modeling of SNG Production in an Entrained-Flow Gasifer Xijia Lu* and Ting Wang Energy Conversion& Conservation Center University of New Orleans New Orleans, LA 70148, USA Abstract In this paper, the coal-to-synthetic natural gas (SNG) 1 Introduction technologies have been reviewed. Steam-oxygen gasification, SNG has a large potential market, particularly for countries hydrogasification, and catalytic steam gasification are the three that don't have domestic natural gas (NG) resources and relies major gasification processes used in coal-to-SNG production. on importing expensive foreign Liquefied Nature Gas (LNG). So far, only the steam-oxygen gasification process is Basically any application that currently uses natural gas could commercially proven by installing a catalytic methanation use SNG. In particular, gasification can be used on-site for reactor downstream of the gasification process after syngas is industrial applications to produce SNG, allowing continued produced, cleaned, and shifted to achieve an appropriate H2/CO operation of natural gas equipment, but from a coal source. ratio for methanation reaction. This process is expensive, less Other than producing electricity, industrial use of natural gas efficient, and time consuming. Ideally, it will be more effective was about 28% of the total domestic consumption -

Design of Highly Efficient Coal-Based Integrated Gasification Fuel Cell Power Plants
Journal of Power Sources 195 (2010) 5707–5718 Contents lists available at ScienceDirect Journal of Power Sources journal homepage: www.elsevier.com/locate/jpowsour Design of highly efficient coal-based integrated gasification fuel cell power plants Mu LI, Ashok D. Rao ∗, Jacob Brouwer, G. Scott Samuelsen Advanced Power and Energy Program, University of California, Irvine, CA 92697-3550, USA article info abstract Article history: Integrated gasification fuel cell (IGFC) technology combining coal gasification and solid oxide fuel cell Received 26 January 2010 (SOFC) is believed to be the only viable solution to achieving U.S. Department of Energy (DOE)’s per- Received in revised form 11 March 2010 formance goal for next generation coal-based power plants, producing electricity at 60% efficiency (coal Accepted 12 March 2010 HHV–AC) while capturing more than 90% of the evolved CO . Achieving this goal is challenging even Available online 19 March 2010 2 with high performance SOFCs; design concepts published to date have not demonstrated this perfor- mance goal. In this work an IGFC system concept consisting of catalytic hydro-gasification, proven Keywords: low-temperature gas cleaning and hybrid fuel cell-gas turbine power block (with SOFC operating at SOFC IGFC about 10 bar) is introduced. The system is demonstrating an electricity efficiency greater than 60% (coal Catalytic hydro-gasification HHV basis), with more than 90% of the carbon present in the syngas separated as CO2 amenable to seques- CO2 capture tration. A unique characteristic of the system is recycling de-carbonized, humidified anode exhaust back to the catalytic hydro-gasifier for improved energy integration. -

Report Biogas and Bio-Syngas Upgrading
Report Biogas and bio-syngas upgrading Carried out by: Danish Technological Institute Laura Bailón Allegue and Jørgen Hinge Kongsvang Allé 29 DK–8000 Aarhus C Date: December 2012 1 Content 1 Summary ...................................................................................................................... 5 2 Introduction ................................................................................................................. 6 3 Biogas composition .................................................................................................... 7 3.1 Biogas quality versus feedstock composition ............................................................. 9 3.2 Biogas quality versus production process ................................................................. 12 4 Biogas quality for energy uses ................................................................................ 15 4.1 Biogas for only heat production .................................................................................. 16 4.2 Biogas to cogeneration systems (CHP) ...................................................................... 17 4.2.1 Biogas for internal combustion engines ................................................................................... 17 4.2.2 Biogas for Stirling engines ....................................................................................................... 18 4.2.3 Biogas for gas turbines and micro-turbines ............................................................................. 18 4.2.4 -

Greatpoint Energy-Lignite Research Council
October 1 , 2009 State of North Dakota The Industrial Commission State Capitol Bismark, North Dakota 58505 ATTN: Lignite Research Program Ladies and Gentlemen: Enclosed please find the Application (the “Application”) of GreatPoint Energy, Inc. (“GreatPoint”) in connection with the Commission’s Lignite Research Program. This letter confirms the commitment of GreatPoint’s management to complete the project as described in the Application if the Commission makes the grant requested. Very truly yours, GREATPOINT ENERGY, INC. By: ______________________________ Daniel P. Goldman Executive Vice President and Chief Financial Officer Hereunto Duly Authorized 0 Applicant: GreatPoint Energy 222 Third St. Suite 2163, Cambridge, MA 02412 617-401-8782 / 617-849-5691 (fax) Principal Investigator: Pattabhi Raman, SVP of R&D Date of Application: October 1, 2009 GreatPoint Energy Lignite Catalytic Hydromethanation Development Project Grant Requested from the North Dakota Lignite Research Council Project Expenses: $7,847,769 Amount Requested: $3,923,884 Grant Deadline: October 1, 2009 1 Table of Contents 1.0 Abstract…………………………………………………………………………………………...Page 3 2.0 Project Summary......………………..……………………………………………………………Page 4 3.0 Project Description…………………..………………………………………………......………Page 10 4.0 Standards of Success………………………………………………………………………..…...Page 25 5.0 Background/Qualifications…………………………………………………………….………...Page 25 6.0 Value to North Dakota………………..…………………………………………………………Page 27 7.0 Management…..……………………..…………………………………………………..………Page 29 8.0 Timetable.……………………………………………………………………………………......Page 32 9.0 Project Budget.………………………………………………………………………………......Page 34 10.0 Matching Funds……………………..…………………………………………………..…..…Page 35 11.0 Tax Liability……………………………………………………………………………..…......Page 38 12.0 Appendices………………………………………………………………………………...…...Page 38 12.1 Confidential Information 12.2 Letter of Support 2 1.0 Abstract GreatPoint Energy (“GreatPoint”) is proposing to advance catalytic hydromethanation of North Dakota lignite to the point of commercial readiness. -

Technical Reviewers' Comments
TECHNICAL REVIEWERS’ COMMENTS LRC-LXVIII(68) –A: “Lignite Catalytic Hydromethanation Development Project” Submitted by: GreatPoint Energy Request for: $3,923,884; Total Project Costs: $7,847,769 Amended Request: Phase I $458,500; Phase II $3,489,079 ($3,923,884) Amended Request: Phase I total $917,000; Phase II $6,978,158 ($7,895,158) Principal Investigator: Pattabhi Raman Project Duration: 17 Months 1. OBJECTIVES The objectives or goals of the proposed project with respect to clarity and consistency with Industrial Commission/Lignite Research Council goals are: 1 - very unclear; 2 - unclear; 3 - clear; 4 - very clear; or 5 - exceptionally clear. Reviewer 09-13 (Rating: 4) GreatPoint Energy has been involved in the development of a proprietary hydromethanation process to produce Synthetic Natural Gas (SNG) from solid fuels. They have developed bench and pilot scale facilities which they operate for purposes of further development of their technology and qualification of potential feedstocks. In this Phase 1 proposal, they propose to begin the process of qualifying North Dakota lignite as a suitable feed for their process. If they are technically successful in developing this technology to a commercial scale and it proves to be competitive with other sources of natural gas and synthetic natural gas, there is a potential for the creation of an additional market for North Dakota lignite as well as additional SNG production facilities in North Dakota that could result in a significant number of new jobs. Reviewer 09-14 (Rating: 3) The GPE revised grant request specifies clearly the potential positive implications of their technology upon North Dakota’s lignite industry – if the research results of Phase I (and later Phase II) confirm the efficacy of this technology. -

Study on the Performance of Nio/Znxzr1−X Catalysts for CO2
RSC Advances View Article Online PAPER View Journal | View Issue Study on the performance of NiO/ZnxZr1Àx catalysts for CO hydrogenation Cite this: RSC Adv., 2020, 10,42790 2 Shuai Chang, †ad Wenshuo Mao,†cd Wei Na,ab Wengui Gao,*ab Gaocheng Qucd and Hua Wangabd The NiO/ZnxZr1Àx (x represents the molar mass of Zn) catalyst was prepared by the impregnation method and tested in CO2 methanation. The activity results show that NiO/Zn0.3Zr0.7 has a higher CO2 conversion rate and methane selectivity than NiO/ZnO and NiO/ZnO–ZrO2. Combined with N2 adsorption– desorption, H2-TPR, CO2-TPD, H2-TPD, XRD, TEM, XPS and FTIR and other characterization methods, the physical and chemical properties of NiO/ZnO–ZrO2 were studied. The incorporation of ZnO into NiO/ZrO2 forms a ZnO–ZrO2 solid solution, and the combination of the solid solution weakens the interaction between NiO and the oxide support, thereby promoting the reduction and dispersion of NiO. Received 7th September 2020 The H -TPR experiment results show that, because ZnO–ZrO forms a solid solution, NiO is better Accepted 14th November 2020 2 2 dispersed on the surface, resulting in a significant reduction in the reduction temperature of NiO. Using Creative Commons Attribution-NonCommercial 3.0 Unported Licence. DOI: 10.1039/d0ra07660k FTIR to conduct CO2 adsorption and methanation experiments on NiO/ZnxZr1Àx to determine the rsc.li/rsc-advances adsorbed species and intermediates, the results show that CO2 methanation follows the formate pathway. 1. Introduction compared to oil and coal, and is safe to use and transport.5–7 Methanation of carbon dioxide is also called the Sabatier reac- 8 Carbon dioxide is the main gas causing the greenhouse effect.