2016 Procedure Targeted Puf User Guide | October 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Utility of the Digital Rectal Examination in the Emergency Department: a Review
The Journal of Emergency Medicine, Vol. 43, No. 6, pp. 1196–1204, 2012 Published by Elsevier Inc. Printed in the USA 0736-4679/$ - see front matter http://dx.doi.org/10.1016/j.jemermed.2012.06.015 Clinical Reviews UTILITY OF THE DIGITAL RECTAL EXAMINATION IN THE EMERGENCY DEPARTMENT: A REVIEW Chad Kessler, MD, MHPE*† and Stephen J. Bauer, MD† *Department of Emergency Medicine, Jesse Brown VA Medical Center and †University of Illinois-Chicago College of Medicine, Chicago, Illinois Reprint Address: Chad Kessler, MD, MHPE, Department of Emergency Medicine, Jesse Brown Veterans Hospital, 820 S Damen Ave., M/C 111, Chicago, IL 60612 , Abstract—Background: The digital rectal examination abdominal pain and acute appendicitis. Stool obtained by (DRE) has been reflexively performed to evaluate common DRE doesn’t seem to increase the false-positive rate of chief complaints in the Emergency Department without FOBTs, and the DRE correlated moderately well with anal knowing its true utility in diagnosis. Objective: Medical lit- manometric measurements in determining anal sphincter erature databases were searched for the most relevant arti- tone. Published by Elsevier Inc. cles pertaining to: the utility of the DRE in evaluating abdominal pain and acute appendicitis, the false-positive , Keywords—digital rectal; utility; review; Emergency rate of fecal occult blood tests (FOBT) from stool obtained Department; evidence-based medicine by DRE or spontaneous passage, and the correlation be- tween DRE and anal manometry in determining anal tone. Discussion: Sixteen articles met our inclusion criteria; there INTRODUCTION were two for abdominal pain, five for appendicitis, six for anal tone, and three for fecal occult blood. -

The Differences Between ICD-9 and ICD-10
Preparing for the ICD-10 Code Set: Fact Sheet 2 October 1, 2015 Compliance Date Get the Facts to be Compliant Alert: The new ICD-10 compliance date is October 1, 2015. The Differences Between ICD-9 and ICD-10 This is the second fact sheet in a series and is focused on the differences between the ICD-9 and ICD-10 code sets. Collectively, the fact sheets will provide information, guidance, and checklists to assist you with understanding what you need to do to implement the ICD-10 code set. The ICD-10 code sets are not a simple update of the ICD-9 code set. The ICD-10 code sets have fundamental changes in structure and concepts that make them very different from ICD-9. Because of these differences, it is important to develop a preliminary understanding of the changes from ICD-9 to ICD-10. This basic understanding of the differences will then identify more detailed training that will be needed to appropriately use the ICD-10 code sets. In addition, seeing the differences between the code sets will raise awareness of the complexities of converting to the ICD-10 codes. Overall Comparisons of ICD-9 to ICD-10 Issues today with the ICD-9 diagnosis and procedure code sets are addressed in ICD-10. One concern today with ICD-9 is the lack of specificity of the information conveyed in the codes. For example, if a patient is seen for treatment of a burn on the right arm, the ICD-9 diagnosis code does not distinguish that the burn is on the right arm. -
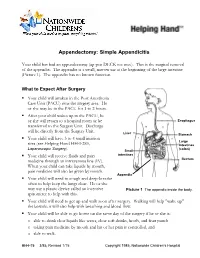
Appendectomy: Simple Appendicitis
Appendectomy: Simple Appendicitis Your child has had an appendectomy (ap pen DECK toe mee). This is the surgical removal of the appendix. The appendix is a small, narrow sac at the beginning of the large intestine (Picture 1). The appendix has no known function. What to Expect After Surgery . Your child will awaken in the Post Anesthesia Care Unit (PACU) near the surgery area. He or she may be in the PACU for 1 to 2 hours. After your child wakes up in the PACU, he or she will return to a hospital room or be Esophagus transferred to the Surgery Unit. Discharge will be directly from the Surgery Unit. Liver Stomach . Your child will have 3 to 4 small incision Large sites (see Helping Hand HH-I-283, Intestines Laparoscopic Surgery (colon) ). Small . Your child will receive fluids and pain intestines medicine through an intravenous line (IV). Rectum When your child can take liquids by mouth, pain medicine will also be given by mouth. Appendix . Your child will need to cough and deep-breathe often to help keep the lungs clear. He or she may use a plastic device called an incentive Picture 1 The appendix inside the body. spirometer to help with this. Your child will need to get up and walk soon after surgery. Walking will help "wake up" the bowels; it will also help with breathing and blood flow. Your child will be able to go home on the same day of the surgery if he or she is: o able to drink clear liquids like water, clear soft drinks, broth, and fruit punch o taking pain medicine by mouth and his or her pain is controlled, and o able to walk. -

Pancreaticoduodenectomy with Preservation of Gastric Tube Blood
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 5 (2014) 746–749 Contents lists available at ScienceDirect International Journal of Surgery Case Reports j ournal homepage: www.casereports.com Pancreaticoduodenectomy with preservation of gastric tube blood flow after esophagectomy: Report of a case ∗ Sho Okimoto, Tsuyoshi Kobayashi , Shintaro Kuroda, Hiroyuki Tahara, Masahiro Ohira, Kentaro Ide, Kohei Ishiyama, Hirotaka Tashiro, Hideki Ohdan Department of Gastroenterological and Transplant Surgery, Applied Life Sciences, Institute of Biomedical & Health Sciences, Hiroshima University, 734-8551, 1-2-3, Kasumi, Hiroshima, Japan a r a t b i c l e i n f o s t r a c t Article history: INTRODUCTION: During pancreaticoduodenectomy (PD), the gastroduodenal artery (GDA) is commonly Received 10 July 2014 divided. In this study, we described the clinical features of PD in which the GDA was preserved in order Received in revised form 5 August 2014 to avoid gastric tube ischemia in a patient who had previously undergone esophagectomy. Accepted 7 August 2014 PRESENTATION OF CASE: A 70-year-old man had previously undergone esophagectomy. Esophagectomy Available online 28 August 2014 and gastric tube reconstruction were performed 10 years earlier due to superior thoracic esophageal can- cer. The patient was referred to our hospital for the treatment of obstructive jaundice and was diagnosed Keywords: with middle bile duct cancer. We performed PD and preserved the GDA. The postoperative course was Bile duct cancer Pancreaticoduodenectomy uneventful, and the gastric tube continued functioning well. DISCUSSION: In a patient with a prior esophagectomy and gastric tube reconstruction, the blood flow to Gastroduodenal artery Esophagectomy the gastric tube is supplied only by the GDA via the right gastroepiploic artery (RGEA). -

About Liver Resection
ABOUT LIVER RESECTION Surgical removal of part of the liver A guide for patients and relatives This booklet has been written to provide information about the operation called a liver resection. This is a major operation and involves removal of a part of the liver. Information about the benefits and risks will help you make an informed decision about the operation. It is important to remember that each person is different. This booklet cannot replace the professional advice and expertise of a doctor who is familiar with your condition. If you have questions that this booklet does not cover, please discuss them with your surgeon or cancer nurse specialist. page 2 What is the liver? The liver is a large organ which lies on the right side of the upper abdomen, under the rib cage. It has many functions related to body metabolism (chemical processes within the body) and is very important to health. One of its functions is to produce yellow-green fluid called bile. Bile flows down a tube called the bile duct to the intestine, where it mixes with food and helps digestion. The gall bladder is a small sac attached to the side of the bile duct. The gall bladder stores excess bile and pushes it down the bile duct in to the intestine, ready for when it is needed for digestion. The liver has right and left lobes (sections). An artery (hepatic artery) and a vein (portal vein) carry blood to the liver. Blood from the liver flows through the hepatic veins back to the heart. -

Thoraco-Laparoscopic Esophagectomy: Thoracic Stage in Prone Position
DOI: 10.1590/0100-69912017005002 Original Article Thoraco-laparoscopic esophagectomy: thoracic stage in prone position Esofagectomia vídeo-tóraco-laparoscópica com tempo torácico em posição pronada CARLOS BERNARDO COLA, TCBC-RJ1,2, FLÁVIO DUARTE SABINO, TCBC-RJ1, CARLOS EDUARDO PINTO, TCBC-RJ1, MARIA RIBEIRO MORARD, TCBC-RJ2, PEDRO PORTARI FILHO, TCBC-RJ2, TEREZA GUEDES1. ABSTRACT Objective: to analyze the National Cancer Institute Abdominopelvic Division (INCA / MS/HC I) initial experience with thoraco-laparoscopic esophagectomy with thoracic stage in prone position. Methods: we studied 19 consecutive thoraco-laparoscopic esophagectomies from may 2012 to august 2014, including ten patients with squamous cells carcinoma (five of the middle third and five of the lower third) and nine cases of gastroesophageal junction adenocarcinoma (six Siewert I and three Siewert II). All procedures were initiated by the prone thoracic stage. Results: There were minimal blood loss, optimal mediastinal visualization, oncological radicality and no conversions. Surgical morbidity was 42 %, most being minor complications (58% Clavien I or II), with few related to the technique. The most common complica- tion was cervical anastomotic leak (37%), with a low anastomotic stricture rate (two stenosis: 10.53%). We had one (5.3%) surgical related death, due to a gastric tube`s mediastinal leak, treated by open reoperation and neck diversion. The median Intensive Care Unit stay and hospital stay were two and 12 days, respectively. The mean thoracoscopic stage duration was 77 min. Thirteen patients received neoadju- vant treatment (five squamous cells carcinoma and eight gastroesophageal adenocarcinomas). The average lymph node sample had 16.4 lymph nodes per patient and 22.67 when separately analyzing patients without neoadjuvant treatment. -
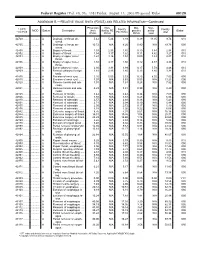
RELATIVE VALUE UNITS (RVUS) and RELATED INFORMATION—Continued
Federal Register / Vol. 68, No. 158 / Friday, August 15, 2003 / Proposed Rules 49129 ADDENDUM B.—RELATIVE VALUE UNITS (RVUS) AND RELATED INFORMATION—Continued Physician Non- Mal- Non- 1 CPT/ Facility Facility 2 MOD Status Description work facility PE practice acility Global HCPCS RVUs RVUs PE RVUs RVUs total total 42720 ....... ........... A Drainage of throat ab- 5.42 5.24 3.93 0.39 11.05 9.74 010 scess. 42725 ....... ........... A Drainage of throat ab- 10.72 N/A 8.26 0.80 N/A 19.78 090 scess. 42800 ....... ........... A Biopsy of throat ................ 1.39 2.35 1.45 0.10 3.84 2.94 010 42802 ....... ........... A Biopsy of throat ................ 1.54 3.17 1.62 0.11 4.82 3.27 010 42804 ....... ........... A Biopsy of upper nose/ 1.24 3.16 1.54 0.09 4.49 2.87 010 throat. 42806 ....... ........... A Biopsy of upper nose/ 1.58 3.17 1.66 0.12 4.87 3.36 010 throat. 42808 ....... ........... A Excise pharynx lesion ...... 2.30 3.31 1.99 0.17 5.78 4.46 010 42809 ....... ........... A Remove pharynx foreign 1.81 2.46 1.40 0.13 4.40 3.34 010 body. 42810 ....... ........... A Excision of neck cyst ........ 3.25 5.05 3.53 0.25 8.55 7.03 090 42815 ....... ........... A Excision of neck cyst ........ 7.07 N/A 5.63 0.53 N/A 13.23 090 42820 ....... ........... A Remove tonsils and ade- 3.91 N/A 3.63 0.28 N/A 7.82 090 noids. -

Incidental Drainage of a Periappendicular Abscess During Colonoscopy
UCTN – Unusual cases and technical notes E175 Incidental drainage of a periappendicular abscess during colonoscopy A 50-year-old man was referred to the of oral metronidazole and ciprofloxacin. A P. Figueiredo, V. Fernandes, J. Freitas outpatient colonoscopy clinic after a posi- computed tomography (CT) scan 1 week Department of Gastroenterology, tive fecal occult blood test during screen- after the procedure revealed no abnormal Hospital Garcia de Orta, Almada, Portugal ing for colorectal cancer. Colonoscopy, findings and the patient remained asymp- which was performed with the patient tomatic. sedated, revealed a 12-mm tumor covered Acute appendicitis is the most frequent References by normal, smooth mucosa at the site of acute abdominal emergency seen in de- 1 Oliak D, Yamini D, Udani VM et al. Can per- forated appendicitis be diagnosed preopera- the appendicular orifice. A biopsy was veloped countries. Its most common com- tively based on admission factors? J Gastro- taken, but this led to an immediate puru- plication is perforation and this may be intest Surg 2000; 4: 470–474 lent discharge occurring from the lesion followed by abscess formation [1]. Colo- 2 Ohtaka M, Asakawa A, Kashiwagi A et al. (●" Video 1). Therefore, a diagnosis of a noscopic diagnosis and treatment of a Pericecal appendiceal abscess with drainage periappendicular abscess was incidentally periappendicular abscess is rare [2]. In during colonoscopy. Gastrointest Endosc 1999; 49: 107–109 established. this case a periappendicular abscess was 3 Antevil J, Brown C. Percutaneous drainage After the patient had recovered from the incidentally discovered and drained dur- and interval appendectomy. In: Scott-Turner sedation, he was specifically questioned ing a colonoscopy. -

Immune Functions of the Vermiform Appendix
The Proceedings of the International Conference on Creationism Volume 3 Print Reference: Pages 335-342 Article 30 1994 Immune Functions of the Vermiform Appendix Frank Maas Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Maas, Frank (1994) "Immune Functions of the Vermiform Appendix," The Proceedings of the International Conference on Creationism: Vol. 3 , Article 30. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol3/iss1/30 IMMUNE FUNCTIONS OF THE VERMIFORM APPENDIX FRANK MAAS, M.S. 320 7TH STREET GERVAIS, OR 97026 KEYWORDS Mucosal immunology, gut-associated lymphoid tissues. immunocompetence, appendix (human and rabbit), appendectomy, neoplasm, vestigial organs. ABSTRACT The vermiform appendix Is purported to be the classic example of a vestigial organ, yet for nearly a century it has been known to be a specialized organ highly infiltrated with lymphoid tissue. This lymphoid tissue may help protect against local gut infections. As the vertebrate taxonomic scale increases, the lymphoid tissue of the large bowel tends to be concentrated In a specific region of the gut: the cecal apex or vermiform appendix. -

Appendicitis
Appendicitis National Digestive Diseases Information Clearinghouse The appendix is a small, tube-like structure abdomen. Anyone can get appendicitis, attached to the first part of the large intes- but it occurs most often between the ages tine, also called the colon. The appendix of 10 and 30. is located in the lower right portion of National Institute of the abdomen. It has no known function. Diabetes and Removal of the appendix appears to cause Causes Digestive The cause of appendicitis relates to block- and Kidney no change in digestive function. Diseases age of the inside of the appendix, known Appendicitis is an inflammation of the as the lumen. The blockage leads to NATIONAL INSTITUTES appendix. Once it starts, there is no effec- increased pressure, impaired blood flow, OF HEALTH tive medical therapy, so appendicitis is and inflammation. If the blockage is not considered a medical emergency. When treated, gangrene and rupture (breaking treated promptly, most patients recover or tearing) of the appendix can result. without difficulty. If treatment is delayed, the appendix can burst, causing infection Most commonly, feces blocks the inside and even death. Appendicitis is the most of the appendix. Also, bacterial or viral common acute surgical emergency of the infections in the digestive tract can lead to Inflamed appendix Small intestine Appendix Large intestine U.S. Department The appendix is a small, tube-like structure attached to the first part of the large intestine, also called the colon. The of Health and appendix is located in the lower right portion of the abdomen, near where the small intestine attaches to the large Human Services intestine. -

Liver Resections Combined with Closure of Loop Ileostomies: a Retrospective Analysis
Hindawi Publishing Corporation HPB Surgery Volume 2008, Article ID 501397, 5 pages doi:10.1155/2008/501397 Research Article Liver Resections Combined with Closure of Loop Ileostomies: A Retrospective Analysis Jeffrey T. Lordan, Angela T. Riga, and Nariman D. Karanjia Regional Hepato-Pancreatico-Biliary Unit for Surrey and Sussex, The Royal Surrey County Hospital, Egerton Road, Guildford, Surrey GU2 7XX, UK Correspondence should be addressed to Jeffrey T. Lordan, dr [email protected] Received 6 August 2008; Accepted 30 October 2008 Recommended by Olivier Farges Background. The management of patients with colorectal liver metastases and loop ileostomies remains controversial. This study was performed to assess the outcome of combined liver resection and loop ileostomy closure. Methods. Analysis of prospectively collected perioperative data, including morbidity and mortality, of 283 consecutive hepatectomies for colorectal liver metastases was undertaken. Consecutive liver resections were performed from 1996 to 2006 in one centre by a single surgeon (NDK). Fourteen of these patients had combined liver resection and ileostomy closure. Case-matched analysis was undertaken. Results.Six(2.2%) patients died in the hepatectomy only group and none died in the combined group. There was no difference in operative blood loss between the two groups (0.09). Perioperative morbidity was 36% in the combined group and 23% in the hepatectomy alone group (P = 0.33). Mean hospital stay was 14 days in the combined group and 11 days in the hepatectomy only group (P = 0.046). Case-matched analysis showed a significant increase in hospital stay (P = 0.03) and complications (P = 0.049) in the combined group. -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance.......................................................................................................................