Published Version
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Different Pockmark Systems and Their Potential Importance for the Hydrological and Biogeochemical Balance of a Perialpine Lake
Different pockmark systems and their potential importance for the hydrological and biogeochemical balance of a perialpine lake Adeline N.Y. Cojean*, Maciej Bartosiewicz**, Jeremy Zimmermann*, Moritz F. Lehmann**, Katrina Kremer*** and Stefanie B. Wirth* * Centre for Hydrogeology and Geothermics, University of Neuchatel, Rue Emile-Argand 11, CH-2000 Neuchâtel ([email protected]) ** Department of Environmental Sciences, University of Basel, Bernoullistrasse 30, CH-4056 Basel ***Swiss Seismological Service (SED), ETH Zürich, Sonneggstrasse 5, CH-8006 Zürich Lacustrine pockmarks Ø Much less investigated than marine pockmark systems Ø Fluid-flow formation Ø CH4 gas ebullition => Lake Constance (Wessel 2010; Bussmann, 2011) Ø Groundwater discharge => Lake Neuchâtel (Reusch 2015; Wirth et al., in prep.) Pockmarks in Lake Thun, Switzerland Thun Lake Thun Tannmoos Fault gypsum carying bedrock Einigen Fault Zone Spiez Fabbri et al., 2017 Beatenberg Interlaken Research questions Thun Ø Are there more pockmarks in Lake Lake Thun Thun? Ø If yes, where are they? Ø What is their mechanism of formation? Spikes in electrical Taanmoos conductivity Ø What is their influence on the lake hydrological and biogeochemical Einigen budget? Fault Zone Beatenberg karst system Spiez Beatenberg Beaten Connected to Daerligen karst system Interlaken Intensive CH4 bubbling Different pockmarks systems in Lake Thun Thun Lake Thun Connection to karst system leads to groundwater discharge? Tannmoos Einigen Fault Zone Beatenberg karst system Spiez Beatenberg Beaten Daerligen -

The 1996 AD Delta Collapse and Large Turbidite in Lake Brienz ⁎ Stéphanie Girardclos A, , Oliver T
Marine Geology 241 (2007) 137–154 www.elsevier.com/locate/margeo The 1996 AD delta collapse and large turbidite in Lake Brienz ⁎ Stéphanie Girardclos a, , Oliver T. Schmidt b, Mike Sturm b, Daniel Ariztegui c, André Pugin c,1, Flavio S. Anselmetti a a Geological Institute-ETH Zurich, Universitätsstr. 16, CH-8092 Zürich, Switzerland b EAWAG, Überlandstrasse 133, CH-8600 Dübendorf, Switzerland c Section of Earth Sciences, Université de Genève, 13 rue des Maraîchers, CH-1205 Geneva, Switzerland Received 13 July 2006; received in revised form 15 March 2007; accepted 22 March 2007 Abstract In spring 1996 AD, the occurrence of a large mass-transport was detected by a series of events, which happened in Lake Brienz, Switzerland: turbidity increase and oxygen depletion in deep waters, release of an old corpse into surface waters and occurrence of a small tsunami-like wave. This mass-transport generated a large turbidite deposit, which is studied here by combining high- resolution seismic and sedimentary cores. This turbidite deposit correlates to a prominent onlapping unit in the seismic record. Attaining a maximum of 90 cm in thickness, it is longitudinally graded and thins out towards the end of the lake basin. Thickness distribution map shows that the turbidite extends over ∼8.5 km2 and has a total volume of 2.72⁎106 m3, which amounts to ∼8.7 yr of the lake's annual sediment input. It consists of normally graded sand to silt-sized sediment containing clasts of hemipelagic sediments, topped by a thin, white, clay-sized layer. The source area, the exact dating and the possible trigger of this turbidite deposit, as well as its flow mechanism and ecological impact are presented along with environmental data (river inflow, wind and lake-level measurements). -

Human Impact on the Transport of Terrigenous and Anthropogenic Elements to Peri-Alpine Lakes (Switzerland) Over the Last Decades
Aquat Sci (2013) 75:413–424 DOI 10.1007/s00027-013-0287-6 Aquatic Sciences RESEARCH ARTICLE Human impact on the transport of terrigenous and anthropogenic elements to peri-alpine lakes (Switzerland) over the last decades Florian Thevenon • Stefanie B. Wirth • Marian Fujak • John Pote´ • Ste´phanie Girardclos Received: 22 August 2012 / Accepted: 6 February 2013 / Published online: 22 February 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract Terrigenous (Sc, Fe, K, Mg, Al, Ti) and suspended sediment load at a regional scale. In fact, the anthropogenic (Pb and Cu) element fluxes were measured extensive river damming that occurred in the upstream in a new sediment core from Lake Biel (Switzerland) and watershed catchment (between ca. 1930 and 1950 and up to in previously well-documented cores from two upstream 2,300 m a.s.l.) and that significantly modified seasonal lakes (Lake Brienz and Lake Thun). These three large peri- suspended sediment loads and riverine water discharge alpine lakes are connected by the Aare River, which is the patterns to downstream lakes noticeably diminished the main tributary to the High Rhine River. Major and trace long-range transport of (fine) terrigenous particles by the element analysis of the sediment cores by inductively Aare River. Concerning the transport of anthropogenic coupled plasma mass spectrometry (ICP-MS) shows that pollutants, the lowest lead enrichment factors (EFs Pb) the site of Lake Brienz receives three times more terrige- were measured in the upstream course of the Aare River at nous elements than the two other studied sites, given by the the site of Lake Brienz, whereas the metal pollution was role of Lake Brienz as the first major sediment sink located highest in downstream Lake Biel, with the maximum val- in the foothills of the Alps. -

Constitution of an Automized Processing Chain to Analyse a Meris Time Series of Swiss Lakes
CONSTITUTION OF AN AUTOMIZED PROCESSING CHAIN TO ANALYSE A MERIS TIME SERIES OF SWISS LAKES Daniel Odermatt a, *, Thomas Heege b, Jens Nieke a, Mathias Kneubühler a and Klaus Itten a a Remote Sensing Laboratories (RSL), Dept. of Geography, University of Zurich, Winterthurerstrasse 190, CH-8050 Zurich, Switzerland - (dodermat, nieke, kneub, itten)@geo.unizh.ch b EOMAP GmbH & Co. KG, Sonderflughafen Oberpfaffenhofen, D-82205 Gilching, Germany - [email protected] KEY WORDS: Inland water, Case II water, lakes, MERIS, water constituents ABSTRACT: The physically based Modular Inversion & Processing System (MIP) is used in an automized processing chain for inland water constituent retrieval from MERIS level 1B data. Preprocessing routines are used to automatically convert the ESA generic data products into MIP input data format. Water/land masking, atmospheric correction and water constituent retrieval are accomplished by simple batch executables from MIP. The accuracy of the constituent retrieval mainly depends on the spectral fit between the image input data and the radiative transfer model results extracted from a database. Therefore, thresholds and initial values for model fitting have to account for all occurring lake specific temporal variations and need careful adjustment. 1. INTRODUCTION Heege, 2000), using the Modular Inversion & Processing System (MIP) (Heege and Fischer, 2004). Monitoring of water quality in lakes is required as an integral part of water resource management, in order to guarantee the In this work, a processing chain to automatically derive water sustainable use of water and to track the effects of constituents from MERIS level 1B data for Swiss lakes is built anthropogenic influences. Simultaneously, adequate monitoring around MIP. -

Subaquatic Slope Instabilities: the Aftermath of River Correction And
1 1 Subaquatic slope instabilities: The aftermath of river correction and 2 artificial dumps in Lake Biel (Switzerland) 3 4 Nathalie Dubois1,2, Love Råman Vinnå 3,5, Marvin Rabold1, Michael Hilbe4, Flavio S. 5 Anselmetti4, Alfred Wüest3,5, Laetitia Meuriot1, Alice Jeannet6,7, Stéphanie 6 Girardclos6,7 7 8 1 Eawag, Swiss Federal Institute of Aquatic Science and Technology, Department of 9 Surface Waters – Research and Management, Dübendorf, Switzerland 10 2 Department of Earth Sciences, ETHZ, Zürich, Switzerland 11 3 Physics of Aquatic Systems Laboratory, Margaretha Kamprad Chair, École 12 Polytechnique Fédérale de Lausanne, Institute of Environmental Engineering, 13 Lausanne, Switzerland 14 4 Institute of Geological Sciences and Oeschger Centre for Climate Change Research, 15 University of Bern, Bern, Switzerland 16 5 Eawag, Swiss Federal Institute of Aquatic Science and Technology, Department of 17 Surface Waters - Research and Management, Kastanienbaum, Switzerland 18 6 Department of Earth Sciences, University of Geneva, Geneva, Switzerland 19 7 Institute for Environmental Sciences, University of Geneva, Geneva, Switzerland 20 21 Corresponding author: Nathalie Dubois, [email protected] 22 23 Associate Editor – Fabrizio Felletti 24 Short Title – Mass transport events linked to river correction 25 This document is the accepted manuscript version of the following article: Dubois, N., Råman Vinnå, L., Rabold, M., Hilbe, M., Anselmetti, F. S., Wüest, A., … Girardclos, S. (2019). Subaquatic slope instabilities: the aftermath of river correction and artificial dumps in Lake Biel (Switzerland). Sedimentology. https://doi.org/10.1111/sed.12669 2 26 ABSTRACT 27 River engineering projects are developing rapidly across the globe, drastically 28 modifying water courses and sediment transfer. -

Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe
Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Lara Ibrahim, M.Sc. aus Mazeraat Assaf, Libanon Berichter: Universitätsprofessor Dr. Andreas Schäffer Prof. Dr. Christoph Schäfers Tag der mündlichen Prüfung: 30. Juli 2015 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar Erklärung Ich versichere, dass ich diese Doktorarbeit selbständig und nur unter Verwendung der angegebenen Hilfsmittel angefertigt habe. Weiterhin versichere ich, die aus benutzten Quellen wörtlich oder inhaltlich entnommenen Stellen als solche kenntlich gemacht zu haben. Lara Ibrahim Aachen, am 18 März 2015 Zusammenfassung Die Zulassung von Pflanzenschutzmitteln in der Europäischen Gemeinschaft verlangt unter anderem eine Abschätzung des Risikos für Organismen in der Umwelt, die nicht Ziel der Anwendung sind. Unvertretbare Auswirkungen auf den Naturhalt sollen vermieden werden. Die ökologische Risikoanalyse stellt die dafür benötigten Informationen durch eine Abschätzung der Exposition der Organismen und der sich daraus ergebenden Effekte bereit. Die Effektabschätzung beruht dabei hauptsächlich auf standardisierten ökotoxikologischen Tests im Labor mit wenigen, oft nicht einheimischen Stellvertreterarten. In diesen Tests werden z. B. Effekte auf das Überleben, das Wachstum und/oder die Reproduktion von Fischen bei verschiedenen Konzentrationen der Testsubstanz gemessen und Endpunkte wie die LC50 (Lethal Concentrations for 50%) oder eine NOEC (No Observed Effect Concentration, z. B. für Wachstum oder Reproduktionsparameter) abgeleitet. Für Fische und Wirbeltiere im Allgemeinen beziehen sich die spezifischen Schutzziele auf das Überleben von Individuen und die Abundanz und Biomasse von Populationen. -

Rote Liste Der Fische (Pisces) Österreichs
Rote Liste der Fische (Pisces) Österreichs Georg Wolfram, Ernst Mikschi*‡ Zusammenfassung Die vorliegende Arbeit folgt den Richtlinien zur Fortschreibung der Roten Liste gefähr- deter Tiere Österreichs (Zulka et al. 2001, Umweltbundesamt-Monographien Band 15, Wien). Die aktuelle Checkliste der Fische Österreichs umfasst 84 Taxa. Im Vergleich zur letzten Roten Liste (Spindler et al. 1997, Umweltbundesamt-Monographien Band 87, Wien) wurden einige Taxa aufgrund aktueller nomenklatorischer und taxonomischer Entwicklungen, auf die gesondert eingegangen wird, neu aufgenommen. Zwei Taxa werden als weltweit ausgestorben (Kategorie EX; Extinct) beurteilt, fünf Taxa sind regional ausgestorben (Kategorie RE; Regionally Extinct). In der Gefährdungs- kategorie „Critically Endangered“ (CR, vom Aussterben bedroht) finden sich sechs Ar- ten, 18 in der Kategorie „Endangered“ (EN; stark gefährdet) und 15 in der Kategorie „Vulnerable“ (VU; gefährdet). Neun Arten stehen auf der Vorwarnliste (Kategorie NT; Near Threatened), 17 weitere Arten, also rund ein Fünftel der aufgenommenen Taxa, sind in Österreich der Kategorie „Least Concern“ (LC; nicht gefährdet) zugeordnet. Für drei Taxa mit äußerst unsicherer taxonomischer Situation reicht die Datenlage für eine Ein- stufung nicht aus (Kategorie DD; Data Deficient). Bei weiteren neun in die Checkliste aufgenommenen Arten handelt es sich um überregional verbreitete und reproduzierende Neozoen, die explizit nicht in die Beurteilung im Rahmen der Roten Liste einbezogen werden (Kategorie NE; Not Evaluated). Aufgrund geänderter Definitionen der Gefährdungskategorien ist die vorliegende Ar- beit mit der Roten Liste von Spindler et al. (1997, Umweltbundesamt-Monographien Band 87, Wien) nur bedingt vergleichbar. Die meisten Unterschiede der Gefährdungs- einstufung sind auf methodische Änderungen oder eine geänderte Datenlage zurückzu- führen. Abstract: Red List of Endangered Fishes (Pisces) in Austria The present paper follows the guidelines for updating the Red Lists of threatened animals of Austria (Zulka et al. -

AHNELT H. 2008. Bestimmungsschlüssel Für Die In
Ahnelt H. 2008 Bestimmungsschlüssel 1 BESTIMMUNGSSCHLÜSSEL FÜR DIE IN ÖSTERREICH VORKOMMENDEN FISCHE HARALD AHNELT Department für Theoretische Biologie, Fakultät für Lebenswissenschaften, Universität Wien, Althanstrasse 14, 1090 Wien [email protected] Online: 10 September 2008 Zitiervorschlag: Ahnelt H. 2008 Bestimmungsschlüssel für die in Österreich vorkommenden Fische. http://homepage.univie.ac.at/harald.ahnelt/Harald_Ahnelts_Homepage/Publications.html [Download-Datum] Bestimmungsschlüssel heimischer Fische Dieser Bestimmungsschlüssel ist für die Fischarten Österreichs ausgelegt. Merkmale und Merkmalskombinationen können daher bei Anwendung auf Fische anderer Länder zu nicht korrekten Ergebnissen führen. Identification key for Austrian freshwater fishes This identification key should only be used for fishes from Austrian freshwaters. This key will possibly not work for fishes from other European countries. Nobody is perfect – schon gar nicht ein Bestimmungsschlüssel. Ein Bestimmungsschlüssel baut auf charakteristischen Merkmalen auf, er vereinfacht und kann keinen Anspruch auf Vollständigkeit erheben. Auch dieser Bestimmungsschlüssel ist nur ein Versuch ein komplexes System in einen übersichtliche Form zu bringen. Die Natur sieht aber oft anders aus. Die Bandbreite an Merkmalen ist bei vielen Arten groß. Manche Populationen sind an unterschiedliche Umweltbedingungen angepasst und bilden unterscheidbare ökologische Formen. Andere Populationen sind isoliert und einige davon sind systematisch noch ungenügend erforscht. Möglicherweise taucht ja in Österreich noch die eine oder andere neue Art auf. Sollte es einmal nicht passen, oder wenn sich ein Fehler eingeschlichen hat, ersuche ich um Information - [email protected] oder unter obiger Adresse. Verbesserungsvorschläge und Ergänzungen sind willkommen. Ahnelt H. 2008 Bestimmungsschlüssel 2 Einleitung 1858 erschien das Buch „Die Süßwasserfische der Österreichischen Monarchie mit Rücksicht auf die angrenzenden Länder“, verfasst von den Österreichern Johann Jakob Heckel und Rudolf Kner. -

Switzerland Vacation
Switzerland Vacation Bruce McKay www.Travel-Pix.ca Switzerland Vacation Contents Contents 2 Introduction 3 Maps 4 Welcome 6 Interlaken 9 Harder Kulm 11 Lauterbrunnen 17 Murren 27 Lake Brienz 36 Schynige Platte 44 Lake Thun 46 Rain Day 50 Zurich 54 Lake Zurich 56 Switzerland Vacation Introduction After I first visited Switzerland and had a great time I often told friends it was a place I'd love to revisit. An opportunity for that arose when I booked on for the European Castles Tours "Three Corners of Europe – Black Forest, Alsace and Switzerland". That tour was designed for air travel to and from Zurich, so there was an easy way to add an extension of my own. The last full day of the tour was at Lucerne, and I was able to plan my extension starting there. I didn't want to repeat everything I'd done before, but I did know there was a magic region where I'd love to spend some more time. The Bernese Oberland in central Switzerland extends south into the Bernese Alps. Its mixture of mountains, lakes, and valleys has made it very popular, not only for sightseeing but also for hiking and active sports, both winter and summer. I visited the most famous sites on an earlier tour. This was a more relaxed visit. I stayed in Interlaken, the regional transportation hub, and made day excursions from there. The photos from my longer tour are in the Switzerland-1 and -2 PDFs, and the ones from the first part of this trip are on the "Three Corners" page. -

Active Faulting in Lake Constance (Austria, Germany, Switzerland) Unraveled by Multi-Vintage Reflection Seismic Data
ORIGINAL RESEARCH published: 11 August 2021 doi: 10.3389/feart.2021.670532 Active Faulting in Lake Constance (Austria, Germany, Switzerland) Unraveled by Multi-Vintage Reflection Seismic Data S.C. Fabbri 1*, C. Affentranger 1, S. Krastel 2, K. Lindhorst 2, M. Wessels 3, Herfried Madritsch 4, R. Allenbach 5, M. Herwegh 1, S. Heuberger 6, U. Wielandt-Schuster 7, H. Pomella 8, T. Schwestermann 8 and F.S. Anselmetti 1 1Institute of Geological Sciences and Oeschger Centre of Climate Change Research, University of Bern, Bern, Switzerland, 2Institute of Geosciences, Christian-Albrechts-Universität zu Kiel, Kiel, Germany, 3Institut für Seenforschung der LUBW, Langenargen, Germany, 4National Cooperative for the Disposal of Radioactive Waste (NAGRA), Wettingen, Switzerland, 5Federal Office of Topography Swisstopo, Wabern, Switzerland, 6Department of Earth Sciences, ETH Zürich, Zürich, Switzerland, 7Landesamt für Geologie, Rohstoffe und Bergbau Baden-Württemberg, Freiburg i. Br., Germany, 8Department of Geology, University of Innsbruck, Innsbruck, Austria Probabilistic seismic hazard assessments are primarily based on instrumentally recorded Edited by: and historically documented earthquakes. For the northern part of the European Alpine Francesco Emanuele Maesano, Istituto Nazionale di Geofisicae Arc, slow crustal deformation results in low earthquake recurrence rates and brings up the Vulcanologia (INGV), Italy necessity to extend our perspective beyond the existing earthquake catalog. The Reviewed by: overdeepened basin of Lake Constance (Austria, Germany, and Switzerland), located Chiara Amadori, within the North-Alpine Molasse Basin, is investigated as an ideal (neo-) tectonic archive. University of Pavia, Italy Alessandro Maria Michetti, The lake is surrounded by major tectonic structures and constrained via the North Alpine University of Insubria, Italy Front in the South, the Jura fold-and-thrust belt in the West, and the Hegau-Lake *Correspondence: Constance Graben System in the North. -

Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change
Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change Jan Baer1, Reiner Eckmann2, Roland Rösch1, Robert Arlinghaus3 & Alexander Brinker1 1Fisheries Research Station, Langenargen, Germany, [email protected] 2University of Konstanz, Germany 3Faculty of Life Sciences, Humboldt-Universität zu Berlin & Department of Biology and Ecology of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany Abstract Upper Lake Constance (ULC) is a large pre-alpine lake situated between Austria, Germany and Switzerland (9°18'E, 47°39'N). Along with the smaller, conjoined expanse of Lower Lake Constance, it forms the third largest lake in Europe. Its waters underwent pronounced eutrophication during the 20th century. Commercial fisheries benefitted strongly from the increased productivity during an initial mesotrophic phase, but these advantages were effectively neutralized when eutrophication became severe. By the turn of the 21st century, internationally coordinated measures to reduce nutrient input to the lake had returned ULC to its historic reference state as an oligotrophic ecosystem. However, the remarkable success of the nutrient management program has been to the detriment of commercial fishers. Yields of most commercially important fish species have decreased, along with lake productivity. As a consequence, the high market demand for local fish products is nowadays met mainly by imports, the ecological footprint of which offsets the local benefits of environmental restoration. Responsibility for fisheries and environmental aspects of ULC managing is shared by the national and federal state administrations and in all cases, tourism, drinking water and environmental interests now take priority over fisheries. -
Print Itinerary
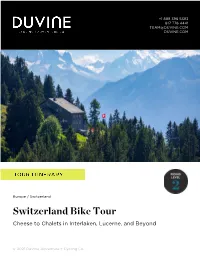
+1 888 396 5383 617 776 4441 [email protected] DUVINE.COM Europe / Switzerland Switzerland Bike Tour Cheese to Chalets in Interlaken, Lucerne, and Beyond © 2021 DuVine Adventure + Cycling Co. Sample Swiss cheese and chocolate right where it’s made Experience the abundance of outdoor landmarks in Interlaken and the jet-set appeal of Gstaad Conquer the Brünig Pass, which links the cities of Interlaken and Lucerne Cycle the shores of Lake Lucerne through quaint communities, Alpine pastures, and vineyards of Riesling and Sauvignon Blanc Arrival Details Departure Details Airport City: Airport City: Geneva, Switzerland Zurich, Switzerland Pick-Up Location: Drop-Off Location: Aigle Train Station Lucerne Train Station Pick-Up Time: Drop-Off Time: 11:30 am 11:00 am NOTE: DuVine provides group transfers to and from the tour, within reason and in accordance with the pick-up and drop-off recommendations. In the event your train, flight, or other travel falls outside the recommended departure or arrival time or location, you may be responsible for extra costs incurred in arranging a separate transfer. Emergency Assistance For urgent assistance on your way to tour or while on tour, please always contact your guides first. You may also contact the Boston office during business hours at +1 617 776 4441 or [email protected]. Travelers Take Note Hiking shoes are recommended for the hike on this tour. We suggest hiking boots or trail shoes with good traction. You may also find moisture-wicking clothing or a daypack useful, but not required. Tour By Day DAY 1 A Very Swiss Beginning Your DuVine guides will greet you in Aigle for a transfer to L'Etivaz, where our tour begins.