Production and Applications of Neutrons Using Particle Accelerators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Concepts for a Deuterium-Deuterium Fusion Reactor
Concepts for a deuterium-deuterium fusion reactor Roberto Onofrio1, 2 1Dipartimento di Fisica e Astronomia “Galileo Galilei”, Universit`adi Padova, Via Marzolo 8, Padova 35131, Italy 2Department of Physics and Astronomy, Dartmouth College, 6127 Wilder Laboratory, Hanover, NH 03755, USA We revisit the assumption that reactors based on deuterium-deuterium (D-D) fusion processes have to be necessarily developed after the successful completion of experiments and demonstrations for deuterium-tritium (D-T) fusion reactors. Two possible mechanisms for enhancing the reactivity are discussed. Hard tails in the energy distribution of the nuclei, through the so-called κ-distribution, allow to boost the number of energetic nuclei available for fusion reactions. At higher temperatures − − than usually considered in D-T plasmas, vacuum polarization effects from real e+e and µ+µ pairs may provide further speed-up due to their contribution to screening of the Coulomb barrier. Furthermore, the energy collection system can benefit from the absence of the lithium blanket, both in simplicity and compactness. The usual thermal cycle can be bypassed with comparable efficiency levels using hadronic calorimetry and third-generation photovoltaic cells, possibly allowing to extend the use of fusion reactors to broader contexts, most notably maritime transport. I. INTRODUCTION high yield scintillating materials and hadronic calorime- try with third-generation photovoltaic cells. This could lead to energy conversion at an initially nearly compara- It is usually assumed that the first commercial fusion ble efficiency with respect to a thermal cycle, with un- reactors will be based on D-T mixtures, and within this known margins for improvement, and within a compact frame a well-defined path has been paved with the ongo- design. -

HISTORY Nuclear Medicine Begins with a Boa Constrictor
HISTORY Nuclear Medicine Begins with a Boa Constrictor Marshal! Brucer J Nucl Med 19: 581-598, 1978 In the beginning, a boa constrictor defecated in and then analyzed the insoluble precipitate. Just as London and the subsequent development of nuclear he suspected, it was almost pure (90.16%) uric medicine was inevitable. It took a little time, but the acid. As a thorough scientist he also determined the 139-yr chain of cause and effect that followed was "proportional number" of 37.5 for urea. ("Propor inexorable (7). tional" or "equivalent" weight was the current termi One June week in 1815 an exotic animal exhibi nology for what we now call "atomic weight.") This tion was held on the Strand in London. A young 37.5 would be used by Friedrich Woehler in his "animal chemist" named William Prout (we would famous 1828 paper on the synthesis of urea. Thus now call him a clinical pathologist) attended this Prout, already the father of clinical pathology, be scientific event of the year. While he was viewing a came the grandfather of organic chemistry. boa constrictor recently captured in South America, [Prout was also the first man to use iodine (2 yr the animal defecated and Prout was amazed by what after its discovery in 1814) in the treatment of thy he saw. The physiological incident was common roid goiter. He considered his greatest success the place, but he was the only person alive who could discovery of muriatic acid, inorganic HC1, in human recognize the material. Just a year earlier he had gastric juice. -

Uses of Isotopic Neutron Sources in Elemental Analysis Applications
EG0600081 3rd Conference on Nuclear & Particle Physics (NUPPAC 01) 20 - 24 Oct., 2001 Cairo, Egypt USES OF ISOTOPIC NEUTRON SOURCES IN ELEMENTAL ANALYSIS APPLICATIONS A. M. Hassan Department of Reactor Physics Reactors Division, Nuclear Research Centre, Atomic Energy Authority. Cairo-Egypt. ABSTRACT The extensive development and applications on the uses of isotopic neutron in the field of elemental analysis of complex samples are largely occurred within the past 30 years. Such sources are used extensively to measure instantaneously, simultaneously and nondestruclively, the major, minor and trace elements in different materials. The low residual activity, bulk sample analysis and high accuracy for short lived elements are improved. Also, the portable isotopic neutron sources, offer a wide range of industrial and field applications. In this talk, a review on the theoretical basis and design considerations of different facilities using several isotopic neutron sources for elemental analysis of different materials is given. INTRODUCTION In principle there are two ways to use neutrons for elemental and isotopic abundance analysis in samples. One is the neutron activation analysis which we call it the "off-line" where the neutron - induced radioactivity is observed after the end of irradiation. The other one we call it the "on-line" where the capture gamma-rays is observed during the neutron bombardment. Actually, the sequence of events occurring during the most common type of nuclear reaction used in this analysis namely the neutron capture or (n, gamma) reaction, is well known for the people working in this field. The neutron interacts with the target nucleus via a non-elastic collision, a compound nucleus forms in an excited state. -

H*(10) Y Fluencias En Un Irradiador De Neutrones Con Una Fuente De Ra-Be
ISSSD 2020 ONLINE _________________________________________________________________________________ H*(10) y fluencias en un irradiador de neutrones con una fuente de 226Ra-Be Bedher O. Vega-Cabrera1,*, Héctor René Vega-Carrillo2, Víctor M. Viera Castillo1, César J. Guevara Pillaca1, Patrizia E., Pereyra Anaya1 María E. López Herrera1, Daniel F. Palacios Fernández1 1Pontificia Universidad Católica del Perú, Sección de Física. Av. Universitaria 1801, Apartado 1761, Lima – Perú. 2Universidad Autónoma de Zacatecas, Unidad Académica de Estudios Nucleares, C. Ciprés 10, Fracc. La Peñuela, 98068 Zacatecas, Zac. México. * E-mail: [email protected] Resumen Un irradiador de neutrones es un moderador con una fuente isotópica que es usado para enseñanza, entrenamiento y actividades de investigación. Normalmente, el moderador tiene puertos de irradiación radial y/o axial. Con el fin de utilizar el irradiador de neutrones de forma segura y óptima, deben conocerse los niveles de dosis y el espectro de la fluencia de neutrones. En este trabajo se utilizaron métodos Monte Carlo para estimar las fluencias de neutrones en tres rangos de energías: térmicos, epitérmicos y rápidos en siete puertos de un irradiador de neutrones con una fuente 226Ra-Be. El irradiador revestido de plomo contiene parafina wax como medio moderador de neutrones y sus puertos están asignados a diferentes distancias de la fuente de neutrones. El equivalente de dosis ambiental, debido a los neutrones, se estimó a 100 cm lateralmente y a 10 cm por encima del irradiador de neutrones. -

Radiation Safety Training
Radiation Safety Training …it concerns your health! 8/30/2006 Charlie Freeman, RSO Slide #1 SUNY Genseseo Atomic Structure Nucleus Orbiting Electrons – Contains Protons and – “Cloud” of orbiting Neutrons electrons surrounds nucleus – Cloud is relatively large – Small Size (~1E-14 m) (~1E-10 m) – Relatively Large Mass – Low mass – Extremely Large Density – Small amount of Stored – Large amount of Stored Energy Energy – Responsible for Chemical Bonds 8/30/2006 Charlie Freeman, RSO Slide #2 SUNY Genseseo Nomenclature • Element Designation A – “X” = Element Symbol X – “Z” = # protons in nucleus Z • “Atomic #” (each element has a unique Z, see periodic For Example table) 16 – “N” = # neutrons 8 O = O-16 = Oxygen 16 – Atomic mass # = “A” 17 • A = Z + N 8 O = O-17 = Oxygen 17 • Isotope: same Z, different N 197 and A 79 Au = Au-197 = Gold 197 8/30/2006 Charlie Freeman, RSO Slide #3 SUNY Genseseo Example: P-32 • 15 protons 32 • 17 neutrons P • A = 32 15 • Z =15 8/30/2006 Charlie Freeman, RSO Slide #4 SUNY Genseseo Ion • In an electrically neutral atom or molecule, the number of electrons equals the number of protons • Any atom or molecule with an imbalance in electrical charge is called an ion • Ions are chemically unstable, and will seek electrical neutrality by reacting with other atoms or molecules. 8/30/2006 Charlie Freeman, RSO Slide #5 SUNY Genseseo Radioactivity • Definition: A collection of unstable atoms that undergo spontaneous transformation that result in new elements. The degree of radioactivity is given by the number of decays that occur per unit time (decays per minute) • Units of measure: – Dpm, dps – Curie (Ci): 1 Ci = 3.7E10 dps – Bequerel (Bq): 1 Bq = 1dps 8/30/2006 Charlie Freeman, RSO Slide #6 SUNY Genseseo Half-Life & Decay Law • The activity of a sample of radioactive 225 200 atoms decreases over 175 150 time 125 100 75 Activity (Curies) • Half-life: how long it 50 25 takes for activity of 0 0 1 2 3 4 5 6 7 sample to decrease by Time (hours) a factor of ½. -

Can Deuterium Formation Be Measured?
CAN DEUTERIUM FORMATION BE MEASURED? Robert K. Soberman∗ and Maurice Dubin† retired (Dated: November 12, 2018) The fundamental PP reaction has never been attempted in the laboratory as theory states it cannot be measured within the human life span. This forms the foundation of stellar nucleosynthesis and the standard solar model. Yet numerous observations including the Sun’s obvious variability argue the theory is flawed and the reaction occurs in times many orders of magnitude shorter than prediction. Considering the consequence to accepted models and controlled fusion, testing is warranted. PACS numbers: 95.30.Cq, 96.60.-j, 25.40.-h There is no record of an attempt to measure the transmutation probability for the fundamental reaction 1H(p, e+,ν)2H. As Bethe and Critchfield [1] predicted a reaction rate of 14(10)9 years no one has been bold enough to test this prediction. Parker and Rolfs [2] wrote “It can be estimated that with a total cross section of 10−47cm2 at Ep(lab) = 1MeV for a proton beam of 1 mA incident on a thick hydrogen target, there would be only one 1H(p, e+,ν)2H reaction in 106 yr.” A similar but numerically discrepant statement appears in Clayton’s classic stellar nucleosynthesis text [3]. As no research team has the patients, resources or longevity to wait out such a prediction it is not surprising that it has never been tested. Such a slow reaction rate was necessary for the standard stellar power model. A much shorter time would cut the lifetime of stars accordingly and a brief reaction time such as seconds would, in that model, cause stars to explode when the reaction conditions were reached. -

Cold Fusion Phenomena May 23-25, 1989 Santa Fe, New Mexico
WORKSHOP ON COLD FUSION PHENOMENA MAY 23-25, 1989 SANTA FE, NEW MEXICO AGENDA Sponsored by Los Alamos National Laboratory and the U.S. Department of Energy TABLE OF CONTENTS Program Committee V Agenda 1-6 Abstracts of Presentations Sessions A - E WORKSHOP CO-CHAIRMAN Prof. Johann Rafelski Department of Physics Dr. Norman Hackerman University of Arizona The Welch Foundation Tucson, AZ Houston, TX Dr. A. John Appleby Dr. J. Robert Schrieffer Center for Electrochemical Systems Director, Institute for and Hydrogen Research Theoretical Physics Texas A&M University University of California College Station, TX Santa Barbara, CA Dr. Anthony L. Turkevich Enrico Fermi Institute TECHNICAL PROGRAM COORDINATOR University of Chicago Chicago, IL Dr. Reed J. Jensen Los Alamos National Laboratory PROGRAM SUPPORT COORDINATOR Mr. David C. Phillips Los Alamos National Laboratory WORKSHOP PROGRAM COMMITTEE Prof. Allen Bard Department of Chemistry University of Texas Austin, TX Dr. Hans Frauenfelder Department of Physics University of Illinois at Urbana-Champaign Urbana, IL Dr. Steven E. Jones Department of Chemistry Brigham Young University Provo, UT Prof. Arthur K. Kerman Director, Laboratory for Nuclear Science MIT Cambridge, MA Prof. Steve Koonin Institute for Theoretical Physics University of California Santa Barbara, CA AGENDA WORKSHOP ON COLD FUSION PHENOMENA May 22-25, 1989 SWEENEY CENTER, SANTA FE, NM MONDAY, MAY 22 Workshop participants arrive in Santa Fe 4:00 - 8:00 Registration and badge issue at EldoradoHotel -Hospitality room available at EldoradoHotel TUESDAY, MAY 23 7:30 - 8:00 Arrival at Sweeney Center 8:00 - 8:30 Late Registration 8:30 - 9:00 Preliminary Remarks Reed J. -

Stellar Evolution
AccessScience from McGraw-Hill Education Page 1 of 19 www.accessscience.com Stellar evolution Contributed by: James B. Kaler Publication year: 2014 The large-scale, systematic, and irreversible changes over time of the structure and composition of a star. Types of stars Dozens of different types of stars populate the Milky Way Galaxy. The most common are main-sequence dwarfs like the Sun that fuse hydrogen into helium within their cores (the core of the Sun occupies about half its mass). Dwarfs run the full gamut of stellar masses, from perhaps as much as 200 solar masses (200 M,⊙) down to the minimum of 0.075 solar mass (beneath which the full proton-proton chain does not operate). They occupy the spectral sequence from class O (maximum effective temperature nearly 50,000 K or 90,000◦F, maximum luminosity 5 × 10,6 solar), through classes B, A, F, G, K, and M, to the new class L (2400 K or 3860◦F and under, typical luminosity below 10,−4 solar). Within the main sequence, they break into two broad groups, those under 1.3 solar masses (class F5), whose luminosities derive from the proton-proton chain, and higher-mass stars that are supported principally by the carbon cycle. Below the end of the main sequence (masses less than 0.075 M,⊙) lie the brown dwarfs that occupy half of class L and all of class T (the latter under 1400 K or 2060◦F). These shine both from gravitational energy and from fusion of their natural deuterium. Their low-mass limit is unknown. -

Primordial Nucleosynthesis in the Precision Cosmology Era the Annual Review of Nuclear and Particle Science Gary Steigman (2007)
Primordial Nucleosynthesis in the Precision Cosmology Era The Annual Review of Nuclear and Particle Science Gary Steigman (2007) Galactic Archeology Seminar Manuel Kramer Universit¨atHeidelberg December 1, 2017 Contents 1 Introduction 2 Fusion of the first nuclides 3 Observations of the relic nuclides 4 Theory vs data 5 Summary Source: CERN Introduction Fusion of the first nuclides Observations of the relic nuclides Theory vs data Summary Fusion of the first nuclides Initial neutron to proton ratio Neutron & Proton equilibrium Once stable hadrons can form from the quark gluon plasma in the very early universe, neutrons and protons are in thermal equilibrium by the weak interactions: − p + e $ n + νe (1) + n + e $ p +ν ¯e Mass of protons is slightly lower than that of the neutrons ni =pi = 1=7 (2) Introduction Fusion of the first nuclides Observations of the relic nuclides Theory vs data Summary Deuterium evolution Deuterium is formed by fusion of a proton and a neutron n + p $ d + γ (3) Significant amounts can only built up when kT . 80 keV −10 The higher the baryon density η10 = 10 (nB=nγ), the higher the interaction rate Introduction Fusion of the first nuclides Observations of the relic nuclides Theory vs data Summary Helium-4 evolution Nucelar fusion processes The starting point for all subsequent fusions is Deuterium Deuterium fusion is a slow reaction Deuterium is immediately converted to 3He and tritium no stable mass-5 nuclides ! bottleneck at 4He Source: https://en.wikipedia.org/ wiki/Big Bang nucleosynthesis Introduction Fusion of -
Modern Physics, the Nature of the Interaction Between Particles Is Carried a Step Further
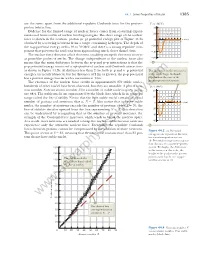
44.1 Some Properties of Nuclei 1385 are the same, apart from the additional repulsive Coulomb force for the proton– U(r ) (MeV) proton interaction. 40 Evidence for the limited range of nuclear forces comes from scattering experi- n–p system ments and from studies of nuclear binding energies. The short range of the nuclear 20 force is shown in the neutron–proton (n–p) potential energy plot of Figure 44.3a 0 r (fm) obtained by scattering neutrons from a target containing hydrogen. The depth of 1 567432 8 the n–p potential energy well is 40 to 50 MeV, and there is a strong repulsive com- Ϫ20 ponent that prevents the nucleons from approaching much closer than 0.4 fm. Ϫ40 The nuclear force does not affect electrons, enabling energetic electrons to serve as point-like probes of nuclei. The charge independence of the nuclear force also Ϫ60 means that the main difference between the n–p and p–p interactions is that the a p–p potential energy consists of a superposition of nuclear and Coulomb interactions as shown in Figure 44.3b. At distances less than 2 fm, both p–p and n–p potential The difference in the two curves energies are nearly identical, but for distances of 2 fm or greater, the p–p potential is due to the large Coulomb has a positive energy barrier with a maximum at 4 fm. repulsion in the case of the proton–proton interaction. The existence of the nuclear force results in approximately 270 stable nuclei; hundreds of other nuclei have been observed, but they are unstable. -

Astronomy General Information
ASTRONOMY GENERAL INFORMATION HERTZSPRUNG-RUSSELL (H-R) DIAGRAMS -A scatter graph of stars showing the relationship between the stars’ absolute magnitude or luminosities versus their spectral types or classifications and effective temperatures. -Can be used to measure distance to a star cluster by comparing apparent magnitude of stars with abs. magnitudes of stars with known distances (AKA model stars). Observed group plotted and then overlapped via shift in vertical direction. Difference in magnitude bridge equals distance modulus. Known as Spectroscopic Parallax. SPECTRA HARVARD SPECTRAL CLASSIFICATION (1-D) -Groups stars by surface atmospheric temp. Used in H-R diag. vs. Luminosity/Abs. Mag. Class* Color Descr. Actual Color Mass (M☉) Radius(R☉) Lumin.(L☉) O Blue Blue B Blue-white Deep B-W 2.1-16 1.8-6.6 25-30,000 A White Blue-white 1.4-2.1 1.4-1.8 5-25 F Yellow-white White 1.04-1.4 1.15-1.4 1.5-5 G Yellow Yellowish-W 0.8-1.04 0.96-1.15 0.6-1.5 K Orange Pale Y-O 0.45-0.8 0.7-0.96 0.08-0.6 M Red Lt. Orange-Red 0.08-0.45 *Very weak stars of classes L, T, and Y are not included. -Classes are further divided by Arabic numerals (0-9), and then even further by half subtypes. The lower the number, the hotter (e.g. A0 is hotter than an A7 star) YERKES/MK SPECTRAL CLASSIFICATION (2-D!) -Groups stars based on both temperature and luminosity based on spectral lines. -

Maximizing Specific Energy by Breeding Deuterium
Maximizing specific energy by breeding deuterium Justin Ball Ecole Polytechnique F´ed´eralede Lausanne (EPFL), Swiss Plasma Center (SPC), CH-1015 Lausanne, Switzerland E-mail: [email protected] Abstract. Specific energy (i.e. energy per unit mass) is one of the most fundamental and consequential properties of a fuel source. In this work, a systematic study of measured fusion cross-sections is performed to determine which reactions are potentially feasible and identify the fuel cycle that maximizes specific energy. This reveals that, by using normal hydrogen to breed deuterium via neutron capture, the conventional catalyzed D-D fusion fuel cycle can attain a specific energy greater than anything else. Simply surrounding a catalyzed D-D reactor with water enables deuterium fuel, the dominant stockpile of energy on Earth, to produce as much as 65% more energy. Lastly, the impact on space propulsion is considered, revealing that an effective exhaust velocity exceeding that of deuterium-helium-3 is theoretically possible. 1. Introduction The aim of this work is to investigate the basic question \What fuel source can provide the most energy with the least mass?" For this question, we will look into the near future and assume that the current incremental and steady development of technology will continue. However, we will not postulate any breakthroughs or new physical processes because they cannot be predicted with any certainty. It is already well-known that the answer to our question must involve fusion fuels [1,2], but nothing more precise has been rigorously establishedz. A more precise answer may be useful in the future arXiv:1908.00834v1 [physics.pop-ph] 2 Aug 2019 when the technology required to implement it becomes available.