(12) Patent Application Publication (10) Pub. No.: US 2005/0249811 A1 Plachetka (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

La Hepatotoxicidad Del Droxicam
Vol. 6, n.º 3 julio - septiembre 1993 butlletí groc Institut Català de Farmacologia Universitat Autònoma de Barcelona La hepatotoxicidad del droxicam Los antiinflamatorios no esteroides (AINE) pue- vía oral a animales de experimentación. 15 Cuando den producir alteraciones hepáticas, que van se disuelve en agua y en medio ácido, así co m o desde aumentos leves, transitorios y subclínicos en el quimo gástrico, se hidroliza inmediatamente de los enzimas hepáticos hasta cuadros bien es- a piroxicam y, según el laboratorio fabricante, se tablecidos de hepatitis citolítica, colostásica o absorbe en forma de piroxicam.16 De hecho, la mixta e, incluso, cuadros de hepatitis crónica. administración de droxicam a animales de labora- to r i o 16 y a seres humanos17 no da lugar a concen- No se conoce exactamente la frecuencia de es- traciones pIasmáticas detectables de droxicam,16 tas reacciones de hepatotoxicidad, pero parece sino de piroxicam. No obstante, se ha podido ser muy baja.1 No obstante, el potencial hepato- comprobar recientemente que tras la administra- tóxico no parece ser el mismo para todos los AINE. En realidad, algunos fueron retirados del ción de droxicam o de piroxicam por vía oral a mercado en los años setenta y ochenta por este ratas, los metabolitos que aparecen en orina no motivo (ibufenac, fenclofenac y benoxaprofeno). son los mismos con ambos fármacos; esto indica- Recientemente en España la Comisión Nacional ría que el metabolismo (probablemente hepático) de Farmacovigilancia recomendó la retirada del no es el mismo y que, por tanto, no se puede mercado del bendazac (Bendalina®), un AINE que, hablar de una equivalencia farmacocinética (y toxi- sin una eficacia clínica bien demostrada, se utili- cológica) absoluta entre los dos fármacos. -

Withdrawing Drugs in the U.S. Versus Other Countries Benson Ninan
Volume 3 | Number 3 Article 87 2012 Withdrawing Drugs in the U.S. Versus Other Countries Benson Ninan Albert I. Wertheimer Follow this and additional works at: http://pubs.lib.umn.edu/innovations Recommended Citation Ninan B, Wertheimer AI. Withdrawing Drugs in the U.S. Versus Other Countries. Inov Pharm. 2012;3(3): Article 87. http://pubs.lib.umn.edu/innovations/vol3/iss3/6 INNOVATIONS in pharmacy is published by the University of Minnesota Libraries Publishing. Commentary POLICY Withdrawing Drugs in the U.S. Versus Other Countries Benson Ninan, Pharm.D.1 and Albert I Wertheimer, PhD, MBA2 1Pharmacy Intern, Rite Aid Pharmacies, Philadelphia, PA and 2Temple University School of Pharmacy, Philadelphia PA Key Words: Drug withdrawals, dangerous drugs, UN Banned Drug list Abstract Since 1979, the United Nations has maintained a list of drugs banned from sale in member countries. Interestingly, there are a number of pharmaceuticals on the market in the USA that have been banned elsewhere and similarly, there are some drug products that have been banned in the United States, but remain on the market in other countries. This report provides a look into the policies for banning drug sales internationally and the role of the United Nations in maintaining the master list for companies and countries to use for local decision guidance. Background recently updated issue is the fourteenth issue, which contains At present, one of the leading causes of death in the U.S. is data on 66 new products with updated/new information on believed to be adverse drug reactions.1-14 More than 20 22 existing products. -

STUDIES with NON-STEROIDAL ANTI-INFLAMMATORY DRUGS By
STUDIES WITH NON-STEROIDAL ANTI-INFLAMMATORY DRUGS by Elizabeth Ann Galbraith M.Sc., C.Biol., M.I.Biol. A thesis submitted for the degree of Doctor of Philosophy in the Faculty of Veterinary Medicine of the University of Glasgow Department of Veterinary Pharmacology M ay 1994 ProQuest Number: 11007888 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11007888 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 4kh! TUT GLASGOW UNIVERSITY ) LIBRARY i To Ian ii TABLE OF CONTENTS Acknowledgements v Declaration vi Summary vii List of tables xi List of figures xv Abbreviations xvii Chapter 1 - General Introduction 1 Chapter 2 - General Material and Methods 29 Chapter 3 - Studies with Flunixin 3.1 Introduction 43 3.2 Experimental Objectives 44 3.3 Materials and Methods 45 3.4 Experiments with Flunixin 48 3.5 Results of Oral Experiments with Flunixin 49 3.6 Results of Intravenous Experiments with Flunixin 53 3.7 Results of Subcutaneous Experiments with Flunixin 55 3.8 Discussion 57 3.9 Tables and Figures -

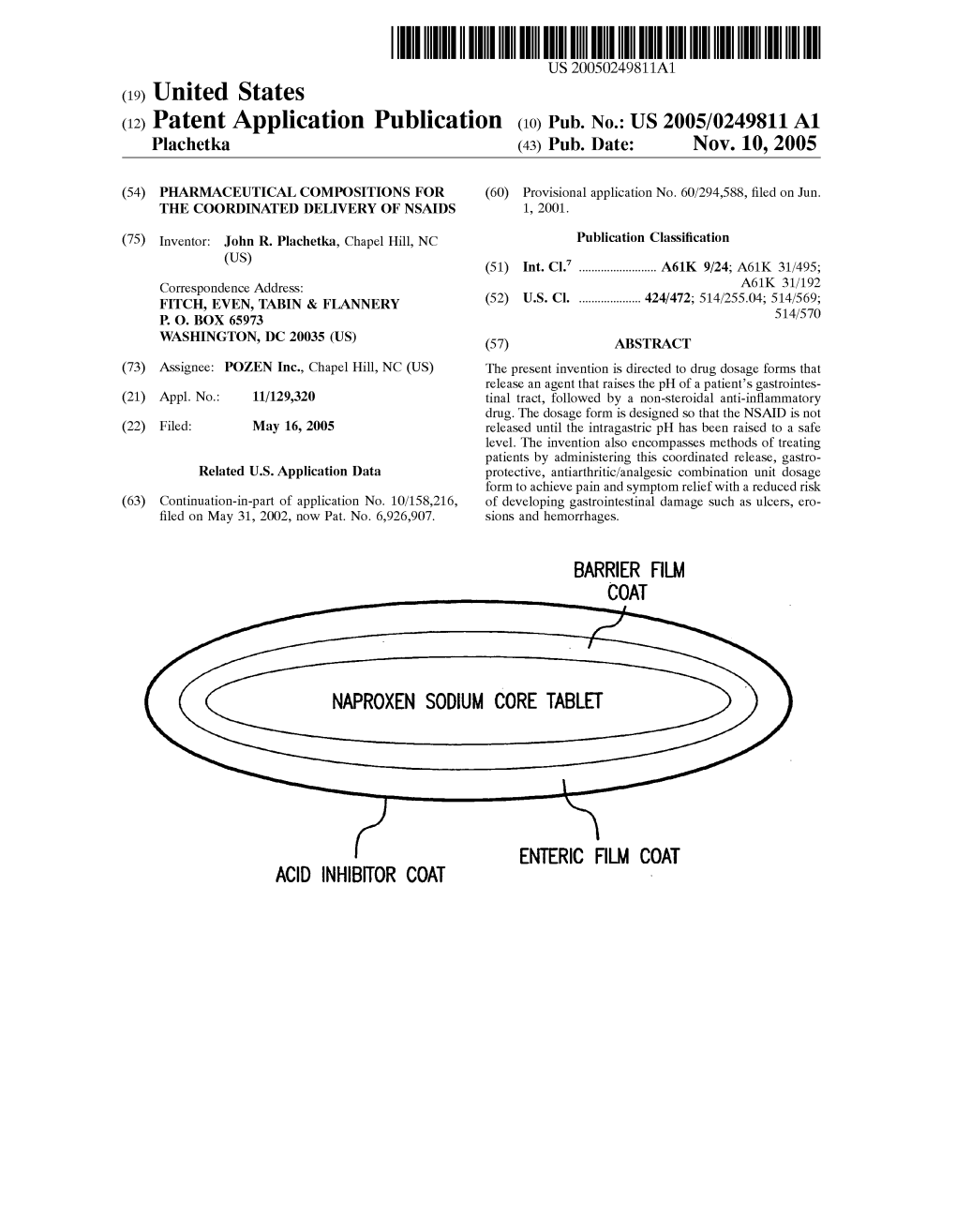
(12) United States Patent (10) Patent No.: US 8,557,285 B2 Plachetka (45) Date of Patent: *Oct
US008557285B2 (12) United States Patent (10) Patent No.: US 8,557,285 B2 Plachetka (45) Date of Patent: *Oct. 15, 2013 (54) PHARMACEUTICAL COMPOSITIONS FOR (56) References Cited THE COORONATED DELVERY OF NSAIDS U.S. PATENT DOCUMENTS (75) Inventor: John R. Plachetka, Chapel Hill, NC 4,198.390 A 4, 1980 Rider ............................ 424/471 (US) 4.255.431 A 3/1981 Junggren et al. .............. 514,338 (73) Assignee: Pozen Inc., Chapel Hill, NC (US) (Continued) (*) Notice: Subject to any disclaimer, the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 AU 2006235929 11, 2006 U.S.C. 154(b) by 0 days. CA 2139653 T 2001 This patent is Subject to a terminal dis (Continued) claimer. OTHER PUBLICATIONS (21) Appl. No.: 13/215,855 Goldstein et al., “PN400 significantly improves upper (22) Filed: Aug. 23, 2011 gastrointestinal tolerability compared with enteric-coated naproxen alone in patients requiring chronic NSAID therapy: Results from (65) Prior Publication Data Two Prospective, Randomized, Controlled Trials.” POZEN Inc. sponsored study, 2009. (Document D15 from Letter to European US 2012/OO64156A1 Mar. 15, 2012 Patent Office for counterpart European Application No. 02734602.2, Related U.S. Application Data regarding Oral Proceedings dated Dec. 18, 2009). (60) Division of application No. 12/553,804, filed on Sep. (Continued) 3, 2009, now abandoned, which is a division of application No. 11/129,320, filed on May 16, 2005, Primary Examiner — Nissa Westerberg now Pat. No. 8,206,741, which is a (74) Attorney, Agent, or Firm — Parker Highlander PLLC continuation-in-part of application No. -

Aspirin Exacerbated Respiratory Disease
Journal of Allergy Aspirin Exacerbated Respiratory Disease Guest Editors: Luis M. Teran, Stephen T. Holgate, Hae-Sim Park, and Anthony P. Sampson Aspirin Exacerbated Respiratory Disease Journal of Allergy Aspirin Exacerbated Respiratory Disease Guest Editors: Luis M. Teran, Stephen T. Holgate, Hae-Sim Park, and Anthony P. Sampson Copyright © 2012 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Journal of Allergy.” All articles are open access articles distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board William E. Berger, USA Marek L. Kowalski, Poland H. Renz, Germany K. Blaser, Switzerland Ting Fan Leung, Hong Kong Nima Rezaei, Iran Eugene R. Bleecker, USA Clare M. Lloyd, UK Robert P. Schleimer, USA J. de Monchy, The Netherlands Redwan Moqbel, Canada Massimo Triggiani, Italy F. Hoebers, The Netherlands Desiderio Passali, Italy Hugo Van Bever, Singapore Stephen T. Holgate, UK Stephen P. Peters, USA A. van Oosterhout, The Netherlands S. L. Johnston, UK David G. Proud, Canada Garry M. Walsh, UK Young J. Juhn, USA F. Rance,´ France Alan P. Knutsen, USA Anuradha Ray, USA Contents Aspirin Exacerbated Respiratory Disease,LuisM.Teran,StephenT.Holgate,Hae-SimPark, and Anthony P. Sampson Volume 2012, Article ID 817910, 2 pages Aspirin Sensitivity and Chronic Rhinosinusitis with Polyps: A Fatal Combination, Hendrik Graefe, Christina Roebke, Dirk Schafer,¨ and Jens Eduard Meyer Volume 2012, Article ID 817910, 10 pages Rhinosinusitis and Aspirin-Exacerbated Respiratory Disease, Maria L. Garcia Cruz, M. Alejandro Jimenez-Chobillon, and Luis M. -

Composition for Use in Treating and Preventing Inflammation Related Disorder
(19) TZZ 54¥¥7A_T (11) EP 2 543 357 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 09.01.2013 Bulletin 2013/02 A61K 9/00 (2006.01) A61K 47/36 (2006.01) (21) Application number: 11173000.8 (22) Date of filing: 07.07.2011 (84) Designated Contracting States: (72) Inventor: Lin, Shyh-Shyan AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei (TW) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: Becker Kurig Straus Designated Extension States: Bavariastrasse 7 BA ME 80336 München (DE) (71) Applicant: Holy Stone Healthcare Co.,Ltd. Taipei City (TW) (54) Composition for use in treating and preventing inflammation related disorder (57) The presentinvention isrelated to ause fortreat- ease, coeliac disease, conjunctivitis, otitis, allergic rhin- ing and preventing inflammation related disorder of a itis, gingivitis, aphthous ulcer, bronchitis, gastroesopha- composition containing a drug and hyaluronic acid (HA) geal reflux disease (GERD), esophagitis, gastritis, en- or HA mixture, whereas the HA or the HA mixture as a teritis, peptic ulcer, inflammatory bowel disease (IBD), delivery vehicle can be a formulation including at least Crohn’s Disease, irritable bowel syndrome (IBS), intes- two HAs having different average molecular weights. The tinal inflammation or allergy, urethritis, cystitis, vaginitis, composition has been demonstrated to be capable of proctitis, eosinophilic gastroenteritis, or rheumatoid ar- reducing the therapeutic dose of a drug on the treatment thritis. and prevention of inflammation related disorders is acute inflammatory disease, chronic obstructed pulmonary dis- EP 2 543 357 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 2 543 357 A1 2 Description alleviate pain by counteracting the cyclooxygenase (COX) enzyme. -

RP-HPLC Method for Determination of Several Nsaids and Their Combination Drugs
Hindawi Publishing Corporation Chromatography Research International Volume 2013, Article ID 242868, 13 pages http://dx.doi.org/10.1155/2013/242868 Research Article RP-HPLC Method for Determination of Several NSAIDs and Their Combination Drugs Prinesh N. Patel, Gananadhamu Samanthula, Vishalkumar Shrigod, Sudipkumar C. Modh, and Jainishkumar R. Chaudhari Department of Pharmaceutical Analysis, National Institute of Pharmaceutical Education and Research (NIPER), Balanagar, Hyderabad, Andhra Pradesh 500037, India Correspondence should be addressed to Gananadhamu Samanthula; [email protected] Received 29 June 2013; Accepted 13 October 2013 Academic Editor: Andrew Shalliker Copyright © 2013 Prinesh N. Patel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. An RP-HPLC method for simultaneous determination of 9 NSAIDs (paracetamol, salicylic acid, ibuprofen, naproxen, aceclofenac, diclofenac, ketorolac, etoricoxib, and aspirin) and their commonly prescribed combination drugs (thiocolchicoside, moxifloxacin, clopidogrel, chlorpheniramine maleate, dextromethorphan, and domperidone) was established. The separation was performed on ∘ Kromasil C18 (250 × 4.6 mm, 5 m) at 35 C using 15 mM phosphate buffer pH 3.25 and acetonitrile with gradient elution ata flow rate of 1.1 mL/min. The detection was performed by a diode array detector (DAD) at 230 nm with total run time of 30min. 2 Calibration curves were linear with correlation coefficients of determinationr ( ) > 0.999. Limit of detection (LOD) and Limit of quantification (LOQ) ranged from 0.04 to 0.97 g/mL and from 0.64 to 3.24 g/mL, respectively. -

Current Prevention and Management of Non-Steroid Anti In.Ammatory
REVIEW ARTICLE &XUUHQW3UHYHQWLRQDQG0DQDJHPHQWRI1RQVWHURLG $QWL,QÀDPPDWRU\'UXJV$VVRFLDWHG*DVWURHQWHURSDWK\ Fransiscus Ari*, Dadang Makmun** *Department of Internal Medicine, Faculty of Medicine, University of Indonesia Dr. Cipto Mangunkusumo General National Hospital, Jakarta **Division of Gastroenterology, Department of Internal Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta ABSTRACT 1RQVWHURLGDQWLLQÀDPPDWRU\GUXJV 16$,'V DUHWKHPRVWIUHTXHQWO\XVHGGUXJVWRWUHDWLQÀDPPDWLRQ and are used almost in the whole world. However, NSAID is one of the important causes of gastroenteropathy development. NSAIDs enteropathy is frequently undetected because most of them are asymptomatic and required sophisticated examinations to diagnose. Not only non-selective cyclo-oxygenases (COX) inhibitor that can cause NSAID gastropathy, but selective COX-2 inhibitors may also cause gastrointestinal complications. NSAID gastroenteropathy require further evaluation and it may differ between patients. Currently, there is no effective treatment available to treat gastrointestinal damage associated with NSAIDs DGPLQLVWUDWLRQ,GHQWL¿FDWLRQRISURWHFWLYHIDFWRUVLQJDVWURLQWHVWLQDOFRPSOLFDWLRQGXHWR16$,'VXVHLVVWLOOD serious challenge. In this review, we will discuss the effect of NSAID administration towards gastrointestinal system, also the prevention and management strategies. Keywords: QRQVWHURLGDQWLLQÀDPPDWRU\GUXJVJDVWURHQWHURSDWK\&2;LQKLELWRUSUHYHQWLRQWUHDWPHQW ABSTRAK 2EDWDQWLLQÀDPDVLQRQVWHURLG 2$,16 DGDODKREDW\DQJSDOLQJVHULQJGLJXQDNDQXQWXNWHUDSLLQÀDPDVL -

Ibuprofen (Oral Route) Description and Brand Names Mayo Clinic
4/6/2017 Ibuprofen (Oral Route) Description and Brand Names Mayo Clinic Drugs and Supplements Ibuprofen (Oral Route) Description and Brand Names Drug information provided by: Micromedex US Brand Name Addaprin Canadian Brand Name Advil Actiprofen AG Profen Descriptions Advil Childrens Bufen Advil Pediatric Genpril Ibuprofen is a nonsteroidal anti Childrens Motrin inflammatory drug (NSAID) used to treat Haltran Childrens Motrin Berry Flavor mild to moderate pain, and helps to Ibu relieve symptoms of arthritis Childrens Motrin Bubble Gum Flavor Ibu2 (osteoarthritis, rheumatoid arthritis, or Childrens Motrin Grape Flavor juvenile arthritis), such as inflammation, Ibu200 Equate Childrens Ibuprofen Berry swelling, stiffness, and joint pain. This Ibu4 Equate Childrens Ibuprofen Berry Dye Freemedicine does not cure arthritis and will Ibu6 help you only as long as you continue to Infants Motrin Ibu8 take it . Personnelle Childrens Ibuprofen Berry Ibuprohm In addition, ibuprofen can be used to Personnelle Childrens Ibuprofen Grape IbuTab treat fever, menstrual cramps, and other conditions as determined by your doctor IPrin . Midol This medicine is available both overthecounter (OTC) and with your doctor's Motrin prescription . Nuprin This product is available in the following dosage forms: Proprinal QProfen Suspension Tablet http://www.mayoclinic.org/drugssupplements/ibuprofenoralroute/description/drg20070602?p=1 1/18 4/6/2017 Ibuprofen (Oral Route) Description and Brand Names Mayo Clinic Capsule, Liquid Filled Tablet, Chewable Capsule Before Using In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. -

Non-Steroidal Anti-Inflammatory Drugs Use in Older Adults Decreases Risk of Alzheimer’S Disease Mortality
RESEARCH ARTICLE Non-steroidal anti-inflammatory drugs use in older adults decreases risk of Alzheimer's disease mortality 1,2,3 4 5 1,2,3 JuliaÂn Benito-Leo nID *, Israel Contador , Saturio Vega , Alberto Villarejo-Galende , FeÂlix Bermejo-Pareja3,6 1 Department of Neurology, University Hospital ª12 de Octubreº, Madrid, Spain, 2 Network Center for Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain, 3 Department of a1111111111 Medicine, Complutense University, Madrid, Spain, 4 Department of Basic Psychology, Psychobiology and a1111111111 Methodology of Behavioural Sciences, University of Salamanca, Salamanca, Spain, 5 AreÂvalo Health Center, a1111111111 AreÂvalo, A vila, Spain, 6 Clinical Research Unit (I+12), University Hospital ª12 de Octubreº, Madrid, Spain a1111111111 * [email protected] a1111111111 Abstract OPEN ACCESS Alzheimer disease (AD) mortality risk in a large cohort of subjects treated or not with non- steroidal anti-inflammatory drugs (NSAIDs) is unknown. Our objective was to determine Citation: Benito-LeoÂn J, Contador I, Vega S, Villarejo-Galende A, Bermejo-Pareja F (2019) Non- whether NSAIDs use is associated with decreased risk of AD mortality. In this prospective, steroidal anti-inflammatory drugs use in older population-based study (Neurological Disorders in Central Spain [NEDICES]) of 5,072 peo- adults decreases risk of Alzheimer's disease ple without AD (aged 65 years and older), sociodemographic, comorbidity factors, and cur- mortality. PLoS ONE 14(9): e0222505. https://doi. org/10.1371/journal.pone.0222505 rent medications were recorded at baseline. Community-dwelling older adults were followed for a median of 12.7 years, after which the death certificates of deceased participants were Editor: Maw Pin Tan, University of Malaya, MALAYSIA examined. -

WO 2013/020527 Al 14 February 2013 (14.02.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/020527 Al 14 February 2013 (14.02.2013) P O P C T (51) International Patent Classification: (74) Common Representative: UNIVERSITY OF VETER¬ A61K 9/06 (2006.01) A61K 47/32 (2006.01) INARY AND PHARMACEUTICAL SCIENCES A61K 9/14 (2006.01) A61K 47/38 (2006.01) BRNO FACULTY OF PHARMACY; University of A61K 47/10 (2006.01) A61K 9/00 (2006.01) Veterinary and Pharmaceutical Sciences Brno Faculty Of A61K 47/18 (2006.01) Pharmacy, Palackeho 1/3, CZ-61242 Brno (CZ). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/CZ20 12/000073 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) Date: International Filing BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 2 August 2012 (02.08.2012) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, 201 1-495 11 August 201 1 ( 11.08.201 1) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 2012- 72 1 February 2012 (01.02.2012) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 2012-5 11 26 July 2012 (26.07.2012) ZW. -

(12) Patent Application Publication (10) Pub. No.: US 2005/0249806A1 Proehl Et Al
US 2005O249806A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0249806A1 Proehl et al. (43) Pub. Date: Nov. 10, 2005 (54) COMBINATION OF PROTON PUMP Related U.S. Application Data INHIBITOR, BUFFERING AGENT, AND NONSTEROIDAL ANTI-NFLAMMATORY (60) Provisional application No. 60/543,636, filed on Feb. DRUG 10, 2004. (75) Inventors: Gerald T. Proehl, San Diego, CA (US); Publication Classification Kay Olmstead, San Diego, CA (US); Warren Hall, Del Mar, CA (US) (51) Int. Cl." ....................... A61K 9/48; A61K 31/4439; A61K 9/20 Correspondence Address: (52) U.S. Cl. ............................................ 424/464; 514/338 WILSON SONS IN GOODRICH & ROSAT (57) ABSTRACT 650 PAGE MILL ROAD Pharmaceutical compositions comprising a proton pump PALO ALTO, CA 94304-1050 (US) inhibitor, one or more buffering agent and a nonsteroidal ASSignee: Santarus, Inc. anti-inflammatory drug are described. Methods are (73) described for treating gastric acid related disorders and Appl. No.: 11/051,260 treating inflammatory disorders, using pharmaceutical com (21) positions comprising a proton pump inhibitor, a buffering (22) Filed: Feb. 4, 2005 agent, and a nonsteroidal anti-inflammatory drug. US 2005/0249806 A1 Nov. 10, 2005 COMBINATION OF PROTON PUMP INHIBITOR, of the Stomach by raising the Stomach pH. See, e.g., U.S. BUFFERING AGENT, AND NONSTEROIDAL Pat. Nos. 5,840,737; 6,489,346; and 6,645,998. ANTI-NFLAMMATORY DRUG 0007 Proton pump inhibitors are typically prescribed for Short-term treatment of active duodenal ulcers, gastrointes CROSS REFERENCE TO RELATED tinal ulcers, gastroesophageal reflux disease (GERD), Severe APPLICATIONS erosive esophagitis, poorly responsive Symptomatic GERD, 0001.