Asian Gypsy Moth EXOTIC PEST THREATS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2
Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) by Boris C. Kondratieff, Paul A. Opler, Matthew C. Garhart, and Jason P. Schmidt C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 March 15, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration (top to bottom): Widow Skimmer (Libellula luctuosa) [photo ©Robert Behrstock], Stonefly (Perlesta species) [photo © David H. Funk, White- lined Sphinx (Hyles lineata) [photo © Matthew C. Garhart] ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences, Colorado State University, Fort Collins, Colorado 80523 Copyrighted 2004 Table of Contents EXECUTIVE SUMMARY……………………………………………………………………………….…1 INTRODUCTION…………………………………………..…………………………………………….…3 OBJECTIVE………………………………………………………………………………………….………5 Site Descriptions………………………………………….. METHODS AND MATERIALS…………………………………………………………………………….5 RESULTS AND DISCUSSION………………………………………………………………………..…...11 Dragonflies………………………………………………………………………………….……..11 -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

Moths of North Carolina - Early Draft 1
Erebidae Spilosoma virginica Virginian Tiger Moth 20 n=9 • • • • • • • High Mt. • • • • N 10 •• • •• • • u • • • • • • • m • • • • • • b • • 0 • e • • • • • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 • 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 51 • • • Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o • 20 • • • f n=50 • = Sighting or Collection Low Mt. High counts of: • • in NC since 2001 F • = Not seen since 2001 l 10 37 - Washington - 1993-08-17 • i 20 - Washington - 1993-05-22 g Status Rank h 17 - Montgomery - 2011-06-29 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 20 20 t n=51 n=156 e Pd CP s 10 10 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Erebidae SUBFAMILY: Arctiinae TRIBE: Arctiini TAXONOMIC_COMMENTS: One of eight species in this genus that occur north of Mexico and one of four species found in North Carolina FIELD GUIDE DESCRIPTIONS: Covell (1984); Beadle and Leckie (2012) ONLINE PHOTOS: MPG, Bugguide, BAMONA TECHNICAL DESCRIPTION, ADULTS: Forbes (1960) TECHNICAL DESCRIPTION, IMMATURE STAGES: Forbes (1960); Wagner (2005) ID COMMENTS: Has nearly all white wings, with usually just a few small black dots on the forewing (often just a dot at the lower angle of the cell) and just one or two spots on the hindwing (Forbes, 1960). -

197 Section 9 Sunflower (Helianthus
SECTION 9 SUNFLOWER (HELIANTHUS ANNUUS L.) 1. Taxonomy of the Genus Helianthus, Natural Habitat and Origins of the Cultivated Sunflower A. Taxonomy of the genus Helianthus The sunflower belongs to the genus Helianthus in the Composite family (Asterales order), which includes species with very diverse morphologies (herbs, shrubs, lianas, etc.). The genus Helianthus belongs to the Heliantheae tribe. This includes approximately 50 species originating in North and Central America. The basis for the botanical classification of the genus Helianthus was proposed by Heiser et al. (1969) and refined subsequently using new phenological, cladistic and biosystematic methods, (Robinson, 1979; Anashchenko, 1974, 1979; Schilling and Heiser, 1981) or molecular markers (Sossey-Alaoui et al., 1998). This approach splits Helianthus into four sections: Helianthus, Agrestes, Ciliares and Atrorubens. This classification is set out in Table 1.18. Section Helianthus This section comprises 12 species, including H. annuus, the cultivated sunflower. These species, which are diploid (2n = 34), are interfertile and annual in almost all cases. For the majority, the natural distribution is central and western North America. They are generally well adapted to dry or even arid areas and sandy soils. The widespread H. annuus L. species includes (Heiser et al., 1969) plants cultivated for seed or fodder referred to as H. annuus var. macrocarpus (D.C), or cultivated for ornament (H. annuus subsp. annuus), and uncultivated wild and weedy plants (H. annuus subsp. lenticularis, H. annuus subsp. Texanus, etc.). Leaves of these species are usually alternate, ovoid and with a long petiole. Flower heads, or capitula, consist of tubular and ligulate florets, which may be deep purple, red or yellow. -

Survey of Lepidoptera of the Wainwright Dunes Ecological Reserve
SURVEY OF LEPIDOPTERA OF THE WAINWRIGHT DUNES ECOLOGICAL RESERVE Alberta Species at Risk Report No. 159 SURVEY OF LEPIDOPTERA OF THE WAINWRIGHT DUNES ECOLOGICAL RESERVE Doug Macaulay Alberta Species at Risk Report No.159 Project Partners: i ISBN 978-1-4601-3449-8 ISSN 1496-7146 Photo: Doug Macaulay of Pale Yellow Dune Moth ( Copablepharon grandis ) For copies of this report, visit our website at: http://www.aep.gov.ab.ca/fw/speciesatrisk/index.html This publication may be cited as: Macaulay, A. D. 2016. Survey of Lepidoptera of the Wainwright Dunes Ecological Reserve. Alberta Species at Risk Report No.159. Alberta Environment and Parks, Edmonton, AB. 31 pp. ii DISCLAIMER The views and opinions expressed are those of the authors and do not necessarily represent the policies of the Department or the Alberta Government. iii Table of Contents ACKNOWLEDGEMENTS ............................................................................................... vi EXECUTIVE SUMMARY ............................................................................................... vi 1.0 Introduction ................................................................................................................... 1 2.0 STUDY AREA ............................................................................................................. 2 3.0 METHODS ................................................................................................................... 6 4.0 RESULTS .................................................................................................................... -

Lepidoptera: Erebidae, Arctiinae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España González, E.; Beccacece, H. M. First record of Dysschema sacrifica (Hübner, [1831]) on Soybean ( Glycine max (L.) Merr) (Lepidoptera: Erebidae, Arctiinae) SHILAP Revista de Lepidopterología, vol. 45, núm. 179, septiembre, 2017, pp. 403-408 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45552790005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Revta. lepid., 45 (179) septiembre 2017: 403-408 eISSN: 2340-4078 ISSN: 0300-5267 First record of Dysschema sacrifica (Hübner, [1831]) on Soybean ( Glycine max (L.) Merr) (Lepidoptera: Erebidae, Arctiinae) E. González & H. M. Beccacece Abstract The presence of Dysschema sacrifica (Hübner, [1831]) on soybean ( Glycine max (L.) Merr) is reported for the first time. Larvae of this species were found consuming soybean leaves in soybean fields in Córdoba province, Argentina, and were able to complete their life cycle. Characteristics of adults and larvae are provided for rapid identification in the field. Due to the widespread distribution of this species within the region where soybean is more intensively cultivated in South America, we conclude that D. sacrifica is a potential soybean pest. Further studies on infestation frequency, damage levels and control by natural enemies are needed. KEY WORDS: Lepidoptera, Erebidae, Arctiidae, Dysschema sacrifica , soybean, pest, Argentina. Primer registro de Dysschema sacrifica (Hübner, [1831]) en soja ( Glycine max (L.) Merr) (Lepidoptera: Erebidae, Arctiinae) Resumen Se reporta por primera vez la presencia de Dysschema sacrifica (Hübner, [1831]) en soja ( Glycine max (L.) Merr). -
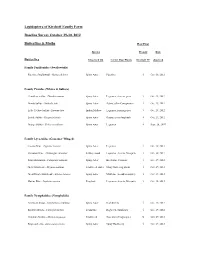
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Reptiles and Amphibians
A good book for beginners is Himmelman’s (2002) book “Discovering Moths’. Winter Moths (2000) describes several methods for By Dennis Skadsen capturing and observing moths including the use of light traps and sugar baits. There are Unlike butterflies, very little fieldwork has a few other essential books listed in the been completed to determine species suggested references section located on composition and distribution of moths in pages 8 & 9. Many moth identification northeast South Dakota. This is partly due guides can now be found on the internet, the to the fact moths are harder to capture and North Dakota and Iowa sites are the most study because most adults are nocturnal, and useful for our area. Since we often identification to species is difficult in the encounter the caterpillars of moths more field. Many adults can only be often than adults, having a guide like differentiated by studying specimens in the Wagners (2005) is essential. hand with a good understanding of moth taxonomy. Listed below are just a few of the species that probably occur in northeast South Although behavior and several physiological Dakota. The list is compiled from the characteristics separate moths from author’s personnel collection, and specimens butterflies including flight periods (moths collected by Gary Marrone or listed in Opler are mainly nocturnal (night) and butterflies (2006). Common and scientific names diurnal (day)); the shapes of antennae and follow Moths of North Dakota (2007) or wings; each have similar life histories. Both Opler (2006). moths and butterflies complete a series of changes from egg to adult called metamorphosis. -

Zootaxa, Virbia & Holomelina (Lepidoptera: Arctiidae: Arctiinae)
ZOOTAXA 1159 Review of generic limits of the tiger moth genera Virbia Walker and Holomelina Herrich-Schäffer (Lepidoptera: Arctiidae: Arctiinae) and their biogeography JENNIFER M. ZASPEL & SUSAN J. WELLER Magnolia Press Auckland, New Zealand JENNIFER M. ZASPEL & SUSAN J. WELLER Review of generic limits of the tiger moth genera Virbia Walker and Holomelina Herrich- Schäffer (Lepidoptera: Arctiidae: Arctiinae) and their biogeography (Zootaxa 1159) 68 pp.; 30 cm. 27 Mar. 2006 ISBN 1-877407-67-4 (paperback) ISBN 1-877407-68-2 (Online edition) FIRST PUBLISHED IN 2006 BY Magnolia Press P.O. Box 41383 Auckland 1030 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2006 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 1159: 1–68 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1159 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) Review of generic limits of the tiger moth genera Virbia Walker and Holomelina Herrich-Schäffer (Lepidoptera: Arctiidae: Arctiinae) and their biogeography JENNIFER M. ZASPEL1,2 & SUSAN J. WELLER1,2,3 1Dept of Entomology, 1980 Folwell Ave, University of Minnesota, St. Paul, MN 55108; E-mail: [email protected] 2Bell Museum of Natural History, 100 Upper Buford Circle, University of Minnesota, St. -

THE MOTHS of JUNE 2020 Slide 1
THE MOTHS Slide 1 OF JUNE 2020 THE MOTHS OF JUNE 2020 Slide 2 The moths described in these slides were found in the morning on the light trap that is in our back yard in London Ontario. My goal is to learn more about my moth neighbours and, by sharing, inspire others to do the same. Light Source Thin White Cotton Sheet Photographer – Eric Auzins PowerPoint – Karen Auzins Species Identification – “Nature Buddies” Descriptions – Wikipedia, BugGuide In Ontario There Are 130 species of Butterflies 2840 Species of Moths Tiger Swallowtail Small-eyed Sphinx There are still moths that are not yet identified! June 6, 2020 Small-eyed Sphinx (Paonias myops) The Small-eyed Sphinx (Paonias myops) is a moth of the family Sphingidae. The hind wings (hidden in this photo) have small "eye" markings. It is found from south-eastern Canada to Florida and westward almost to the Pacific Coast and Mexico. The wingspan is 52–69 mm. Adults are on wing from June to September. The Sphingidae is a family of moths (Lepidoptera), commonly known as hawk moths. They are moderate to large in size and are distinguished among moths for their rapid, sustained flying ability. Their narrow wings and streamlined abdomens are adaptations for rapid flight. Some are capable of flying at over 5.3 m/s (12 miles per hour) The larvae feed on birches, hawthorns, poplars, fruit trees and willows. June 8, 2020 Hickory Tussock Moth (Lophocampa caryae) The Hickory Tussock Moth (Lophocampa caryae) is a member of the family Erebidae which includes the tiger moths. -

Universitätsbibliographie Der Universität Hamburg 2009 Bis 30. Juni 2011 Herausgegeben Von Der Staats- Und Universitätsbibliothek Hamburg Carl Von Ossietzky
Fakultät für Mathematik, Informatik und Naturwissenschaften aus: Universitätsbibliographie der Universität Hamburg 2009 bis 30. Juni 2011 herausgegeben von der Staats- und Universitätsbibliothek Hamburg Carl von Ossietzky S. 747–970 Hamburg University Press Verlag der Staats- und Universitätsbibliothek Hamburg Carl von Ossietzky Impressum Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbib- liografie; detaillierte bibliografische Daten sind im Internet über http://dnb.d-nb.de abrufbar. Die Online-Version dieser Publikation ist auf den Verlagswebseiten frei verfügbar (open access). Die Deutsche Nationalbibliothek hat die Netzpublikation archiviert. Diese ist dauerhaft auf dem Archivserver der Deutschen Nationalbibliothek verfügbar. Open access über die folgenden Webseiten: http://hup.sub.uni-hamburg.de/HamburgUP_Universitaetsbibliographie_2009-2011 Hamburg University Press – http://hup.sub.uni-hamburg.de Archivserver der Deutschen Nationalbibliothek – http://deposit.d-nb.de Universitätsbibliographie online auf den Webseiten der SUB Hamburg: http://www.sub.uni-hamburg.de/ ISBN 978-3-937816-96-8 (Druckausgabe) © 2011 Hamburg University Press, Verlag der Staats- und Universitätsbibliothek Hamburg Carl von Ossietzky, Deutschland Produktion: M+MBlümel GmbH + Co. KG Im Auftrag des Präsidiums der Universität Hamburg. Inhalt Dieter Lenzen Vorwort des Präsidenten der Universität Hamburg VII Gabriele Beger Vorwort der Direktorin der Staats- und Universitätsbibliothek