Nhs Primary & Community Division
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shetland COVID-19 Outbreak Control Plan
Shetland COVID-19 Outbreak Control Plan October 2020 Foreword Our Local Outbreak Plan builds on existing health protection plans and sets out the measures that have been put in place to protect the public’s health and contain any outbreak. It sets out how Shetland Islands Council, NHS Shetland, businesses, voluntary agencies and our local communities are working together to prevent, manage, reduce and suppress outbreaks of COVID-19 in Shetland. It also provides clarity about our individual and collective responsibilities for delivering this plan and responding to an outbreak. A key feature of our plan is the importance of partnership and collaborative working, sharing data and information to make sure we have early warning of any increased transmission, and the established processes we use with all partners to respond quickly for effective management of incidents and outbreaks. The plan also outlines our approach to testing and contact tracing, which is an integral aspect of our strategy to reduce onward transmission of COVID-19. Together we have learned from our experience of COVID early on in the pandemic and worked hard to make sure that our most vulnerable residents are supported to stay safe within their local community. As we enter the next phases of the pandemic, this plan explains how we have and will continue to keep people safe in high risk workplaces, locations and communities. I would like to thank you all for the part you have already played in reducing the virus in our communities. Being vigilant about hygiene, maintaining physical distancing, getting tested when you have symptoms and self-isolating when needed will be an essential part of the way we live our lives for some time to come. -

Emergency Care Weekly Metadata
Publication Metadata (including revision details) Metadata Description Indicator Publication Weekly Update of Emergency Department Activity and Waiting title Times. Description This publication reports key statistics on attendances at Emergency Departments (ED) across Scotland. The information presented in the publication includes trends in the number of attendances and length of time patients spend in ED. Theme Health and Social Care Topic Emergency Care Format Webpage, Excel workbook and CSV. Data source(s) NHS Board aggregate submissions to PHS on Emergency Department Activity and Waiting Times. Date that data Tuesday of the week prior to publication are acquired Release date Every Tuesday Frequency Weekly Timeframe of New data for the week ending 9 days before publication (e.g. 16 data and April publication contains data for week ending 7 April) timeliness Continuity of 1) A&E discharge times at hospitals in NHS Lothian were not data accurately recorded up to November 2017. The Academy of Medical Royal Colleges was commissioned by Scottish Government to ascertain the causes for the data issues in NHS Lothian. The review findings were published 26 June 2018. 2) Since 3 March 2015, the Scottish Government (SG) has released Official Statistics weekly A&E activity and waiting times information for the EDs in Scotland, derived from aggregate information supplied by NHS Boards on the number of attendances and 4, 8, and 12 hour waits. PHS (formally ISD) took over this data collection for statistics covering the week ending 7 June 2015. 3) From 20 May 2018, Raigmore hospital in NHS Highland trialled a new patient flow system. As a consequence the accuracy of some patients’ waits may have been affected between this date and 4 July, however the total number of attendances remains correct. -

Major Players
PUBLIC BODIES CLIMATE CHANGE DUTIES – MAJOR PLAYER ORGANISATIONS Aberdeen City Council Aberdeen City IJB Aberdeenshire Council Aberdeenshire IJB Abertay University Accountant in Bankruptcy Angus Council Angus IJB Argyll and Bute Council Argyll and Bute IJB Audit Scotland Ayrshire College Borders College City of Edinburgh Council City of Glasgow College Clackmannanshire and Stirling IJB Clackmannanshire Council Comhairlie nan Eilean Siar Creative Scotland Disclosure Scotland Dumfries and Galloway College Dumfries and Galloway Council Dumfries and Galloway IJB Dundee and Angus College Dundee City Council Dundee City IJB East Ayrshire Council East Ayrshire IJB East Dunbartonshire Council East Dunbartonshire IJB East Lothian Council Sustainable Scotland Network Edinburgh Centre for Carbon Innovation, High School Yards, Edinburgh, EH1 1LZ 0131 650 5326 ú [email protected] ú www.sustainablescotlandnetwork.org East Lothian IJB East Renfrewshire Council East Renfrewshire IJB Edinburgh College City of Edinburgh IJB Edinburgh Napier University Education Scotland Falkirk Council Falkirk IJB Fife College Fife Council Fife IJB Food Standards Scotland Forth Valley College Glasgow Caledonian University Glasgow City Council Glasgow City IJB Glasgow Clyde College Glasgow Kelvin College Glasgow School of Art Heriot-Watt University The Highland Council Highlands and Islands Enterprise Highlands and Islands Transport Partnership (HITRANS) Historic Environment Scotland Inverclyde Council Inverclyde IJB Inverness College UHI Lews Castle College -

2200022200 Vviiisssiiiooonn
22002200 VViissiioonn ooff SShheettllaanndd’’ss HHeeaalltthhccaarree Fitting together a vision of future health and care services in Shetland NHS Shetland 2020 Vision April 2005 ii NHS SHETLAND 2020 VISION CONTENTS List of Figures & Boxes . iii List of Appendices . iv Acknowledgements . iv Abbreviations . v Executive Summary . vi Section A Introduction & Background 1 A.1 Introduction to NHS Shetland’s 2020 Vision Project . 2 A.2 Strategic Direction for 2020 – outcomes of 2020 Vision Phase 1 . 3 A.3 Introduction to Shetland . 6 A.4 Profile of Shetland Health and Healthcare . 17 A.5 Drivers for change for future Shetland Healthcare . 23 Section B Key Themes for 2020 29 B.1 National Direction . 31 B.2 Shetland Public . 36 B.3 Safety & Quality . 41 B.4 Workforce . 48 B.5 Transport . 59 B.6 Facilities . 67 B.7 Medical Technologies . 71 B.8 Information & Communication Technologies . 75 Section C Shetland Services 2020 81 C.1 Health Improvement . 84 C.2 Disability Services . 95 C.3 Community Health Services . 99 C.4 General Practice . 104 C.5 Mental Health Services . 113 C.6 Dental Services . 117 C.7 Pharmacy Services . 121 C.8 Child Health Services . 124 C.9 Older People’s Services . 131 C.10 Alcohol & Drugs Services . 137 C.11 Clinical Support Services . 144 C.12 Maternity Services . 149 C.13 Hospital Surgical Services . 153 C.14 Hospital Medical Services . 162 C.15 Cancer Services . 170 Section D Our 2020 Vision of Shetland Healthcare 177 Section E Recommendations 185 Appendices . 191 NHS SHETLAND 2020 VISION iii LIST OF FIGURES & BOXES Section A Introduction & Background Box A1 Objectives for Future Healthcare Delivery in Shetland . -

Cross Board AE Data Quality VC 1141119
NHS National Services Scotland Gyle Square 1 South Gyle Crescent EDINBURGH EH12 9EB Telephone 0131 275 6000 http://www.nhsnss.org/ Minutes ISD A&E Data Quality Meeting with NHS Boards Date: Thursday 14th November 2019 Time: 10am Venue: Gyle via TC ISD Attendees: Elaine Pauline, Elizabeth Cole, Katherine McGregor, Renata Walker NHS Board Attendees: NHS Borders: Fiona Kali, Allison Roebuck, Andrea Huggan NHS Grampian: Christine Small, Greg Cook NHS Lothian: Robyn Pascoe, Paul Leonard NHS Orkney: Andrew Marwick NHS Shetland: David Kerr NHS Western Isles: Kathy Jennings, Nicola Walsh, Susan MacAulay, Doreen MacLeod ACTION 1. Welcome and Introductions Elaine welcomed all to the meeting and introductions were made 2. Items for discussion Move to weekly A&E submissions Elaine thanked everyone for the work they have put into the submissions. So far there are no major data issues. She also checked progress towards submitting the new time point data items with each board; NHS Borders Date and Time of First Diagnostics – depends when done, needs to cross match with ED data. Date and Time of Treatment start – not sure, will be a clinician decision if they can get this. Wait to cubicle is possible. Date and Time of Triage– current issue where NHS Borders have got the time seen by first doctor but this can cause an error –as soon as nurse clicks in that field it brings up triage time which can be different. They then need to backdate the time which causes an error. In AAU around 100 patients are seen before triage, which causes 25 errors on the system a day (Triage to seen time comes up as a minus). -

Minutes ISD A&E Data Quality Meeting
NHS National Services Scotland Gyle Square 1 South Gyle Crescent EDINBURGH EH12 9EB Telephone 0131 275 6000 http://www.nhsnss.org/ Minutes ISD A&E Data Quality Meeting with NHS Boards Date: Thursday 15th November 2018 Time: 13.30pm Venue: Gyle via VC ISD Attendees: Elaine Pauline, Elizabeth Cole, Katherine McGregor, Kirstin McNicol NHS Board Attendees: NHS Ayrshire & Arran: Laura Train, Elaine Stewart, Carol Sloan, Katy Purdie & Laura Kelso NHS Fife: Scott Wilson, Daryl Main NHS Grampian: Christine Small, Greg Cook, Hilary Hastings NHS Greater Glasgow & Clyde (GG&C): Kevin Begbie, Brian Reilly MHS Lanarkshire: Pamela Downey, Lorraine Taggart, Laura Morrison, Karen Morrow, Ellie Bates, Lise Axford. NHS Orkney: Gillian Woods NHS Shetland: Stuart Hubbard, Aimee Sutherland ACTION 1. Welcome and Introductions 2. Items for discussion (all) Compliance with 4 hour target/ Questions or concerns over the interpretation of the national A&E guidance Issues raised last year regarding the quality of A&E 4hour waiting times data at NHS Lothian, have led to ISD making changes to their web pages and they will be revising current guidance regarding recording clock stops –this is currently with the SG. ISD are also planning to update the A&E Data Recording manual as it has not been revised for many years. Elaine asked if any of the boards had any questions or concerns regarding complying with the 4 hour target or interpretation of the national A&E guidance. Karen asked for greater clarity regarding nursing home care, and transfer back to the nursing home following A&E attendance. There is ambiguity around types of clinical care - what forms clinical care? – is this medical nurse checking the patient, a medical intervention, a cup of tea being given, or a level of support EP given as the patient is not independent and waiting for transport. -

Scotland's Baby
Scotland’s Baby Box 5th March 2018 Cards Boxes Health Board 19/6-2/3 Received Delivered NHS Ayrshire and Arran 2279 1830 NHS Borders 728 580 NHS Dumfries and Galloway 869 719 NHS Fife 2304 1857 NHS Forth Valley 1891 1564 NHS Grampian 4131 3318 NHS Greater Glasgow and Clyde 9164 7410 NHS Highland 1800 1459 NHS Lanarkshire 3964 3234 NHS Lothian 6399 5182 NHS Orkney 131 110 NHS Shetland 144 115 NHS Tayside 2548 2140 NHS Western Isles 137 110 Total 36489 29628 WC 29/1/18 WC 5/2/18 WC 12/2/18 WC 19/2/18 WC 26/2/18 Cards Received 1049 988 997 1020 663 Orders Confirmed 1187 912 909 941 550 Orders On Hold 80 46 62 49 38 Total Received 19/6-2/3 37152 Total Confirmed 19/6-2/3 35662 Due date by Month 19/6-2/3 Aug-17 2171 Sep-17 3908 Oct-17 3897 Nov-17 3772 Dec-17 3886 Jan-18 3892 Feb-18 3477 Mar-18 3541 Apr-18 3173 May-18 2591 Jun-18 1181 Jul-18 114 Aug-18 1 Total 35604 19/6-2/3 Total Contact for Research 9500 Total Parentclub 8232 Boxes Delivered 29628 Cards Received/Delivered by HB 19/6-2/3 NHS Western Isles 110137 2140 NHS Tayside 2548 NHS Shetland 115 144 NHS Orkney 110 131 5182 NHS Lothian 6399 3234 NHS Lanarkshire 3964 1459 NHS Highland 1800 Boxes Delivered 7410 NHS Greater Glasgow and Clyde 9164 3318 NHS Grampian 4131 Cards Received 1564 NHS Forth Valley 1891 1857 NHS Fife 2304 719 NHS Dumfries and Galloway 869 580 NHS Borders 728 1830 NHS Ayrshire and Arran 2279 0 2000 4000 6000 8000 10000 BABYBOX ORDERS 1400 1200 1187 1049 1020 988 997 1000 912 909 941 800 663 600 550 400 200 80 62 46 49 38 0 WC 29/1/18 WC 5/2/18 WC 12/2/18 WC 19/2/18 WC -

Psychological Therapies Waiting Times in NHS Scotland – Report Publication
Psychological Therapies Waiting Times in NHS Scotland – Report Publication Quarter Ending 31 March 2021 Publication date: 1 June 2021 An Official Statistics release for Scotland Public Health Scotland This is an Official Statistics publication The Official Statistics (Scotland) Order 2008 authorises Public Health Scotland (formerly NHS National Services Scotland (the legal name being the Common Services Agency for the Scottish Health Service)) to produce official statistics. All official statistics should comply with the UK Statistics Authority’s Code of Practice which promotes the production and dissemination of official statistics that inform decision making. They can be formally assessed by the UK Statistics Authority’s regulatory arm for National Statistics status. Find out more about the Code of Practice at: https://www.statisticsauthority.gov.uk/osr/code-of-practice/ Find out more about official statistics at: https://www.statisticsauthority.gov.uk/national-statistician/producers-of-official-statistics/ 2 Public Health Scotland Contents Introduction .............................................................................................................................. 4 Main points ............................................................................................................................... 6 How long people waited to start their treatment (Patients Seen) .......................................... 7 People waiting for treatment at the end of the quarter ....................................................... -

Health in a Changing Scotland
Health IN A CHANGING SCOTLAND The ball’s in our court CALL FOR ABSTRACTS ANNUAL PUBLIC HEALTH CONFERENCE Thursday 6 & Friday 7 November 2014 Macdonald Aviemore Resort Faculty of Public Health Committee of the Faculty of Public Health in Scotland www.fphscotconf.co.uk Announcement of Conference and Call for Abstracts Conference registration Further information regarding registration and accommodation costs will be available in the conference registration brochure which will be circulated in July/August. Please note that acceptance of an abstract Health in a Changing Scotland or shorter presentation application does not provide a free place at the conference. All abstract presenters, including those selected to make oral presentations, are The ball’s in our court required to register in the usual way. A limited number of reduced fee places will be available for delegates unable to secure The title and cover picture for this year’s conference are full funding to attend the conference. The conference plenary sessions will be made intended to reflect 2 key events in 2014 which could have available for those who would like to join the significant implications for Public Health in Scotland – the conference by video-conferencing. Independence Referendum and the Commonwealth Games (although not on the 2014 programme, tennis has long been Conference organisation on the list of approved optional Commonwealth Games sports). By the time of the conference both of these will have This year’s annual Scottish Public Health Conference is being organised by the taken place, and this provides an ideal time to discuss how Faculty of Public Health in partnership with we can proactively use the opportunities that the Games and the North of Scotland Health Boards and Referendum provide to shape the health of our population and comprises the following planning group the structures that support this. -
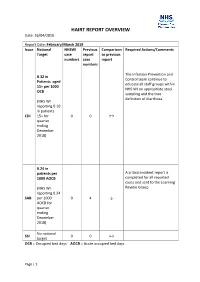
HAIRT REPORT OVERVIEW Date: 26/04/2019
HAIRT REPORT OVERVIEW Date: 26/04/2019 Report Date: February/March 2019 Issue National NHSWI Previous Comparison Required Actions/Comments Target case report to previous numbers case report numbers The Infection Prevention and 0.32 in Control team continue to Patients aged educate all staff groups within 15+ per 1000 NHS WI on appropriate stool OCB sampling and the true definition of diarrhoea. (NHS WI reporting 0.10 in patients CDI 15+ for 0 0 ↔ quarter ending December 2018) 0.24 in patients per A critical incident report is 1000 AOCB completed for all reported cases and sent to the Learning (NHS WI Review Group. reporting 0.24 SAB per 1000 0 4 ↓ AOCB for quarter ending December 2018) No national SSI 0 0 ↔ target OCB = Occupied bed days AOCB = Acute occupied bed days Page | 1 HAIRT REPORT OVERVIEW Figure 1: Funnel plot of CDI incidence rates (per 100,000 TOBD) in healthcare associated infection cases for all NHS Boards in Scotland in Q4 2018. *NHS National Waiting Times Centre, NHS Shetland and NHS Borders overlap as do NHS Highland and NHS Forth Valley. Figure 2: Funnel plot of CDI incidence rates (per 100,000 population) in community associated infection cases for all NHS Boards in Scotland in Q4 2018. *NHS Shetland and NHS Western Isles overlap. Page | 2 HAIRT REPORT OVERVIEW Figure 3: Funnel plot of SAB incidence rates (per 100,000 TOBD) in healthcare associated infection cases for all NHS Boards in Scotland in Q4 2018. *NHS Highland and NHS Forth Valley overlap as do NHS Ayrshire & Arran and NHS Tayside. -

Staff Governance Standard 4Th Edition
Appendix-2012-71 Borders NHS Board STAFF GOVERNANCE STANDARD 4TH EDITION Aim To inform the Board of the national and local launch of the latest version of the Staff Governance Standard Background The NHS Reform (Scotland) Act 2004 requires NHS Scotland employers to deliver the key strategic agenda of ensuring the fair and effective management of staff. The Staff Governance Standard sets out what each NHS Scotland employer must achieve to continuously improve in relation to the fair and effective management of staff A review of the Staff Governance Standard, 3rd edition, was commissioned by The Scottish Workforce and Staff Governance Committee (SWAG) in the summer of 2011. The review was carried out in partnership by a review group comprising representatives of employers, trade unions and professional organisations and the Scottish Government. The new Staff Governance Standard was developed to take into account developments within NHS Scotland, including the implementation of the Healthcare Quality Strategy for Scotland, the three Quality Ambitions and Quality Outcomes, the Patient Rights (Scotland) Act 2011 and the Strategic Narrative setting out our 20:20 Vision for healthcare. The new Staff Governance Standard recognises the importance of a motivated and engaged workforce, with the necessary knowledge and skills, to deliver high quality, person-centred, safe and effective patient care. An important development within this version of the Framework is the introduction of staff responsibilities to complement the employer responsibilities. The new Staff Governance Standard will also form an integral part of the 20:20 workforce vision which is currently in preparation. Summary Within NHS Borders we are currently developing a campaign to launch the standard using resources released from the Scottish Government Health Department. -

Everyone Matters Pulse Survey National Report 2020
Health and Social Care Everyone Matters Pulse Survey National Report 2020 Page 0 of 91 Everyone Matters Pulse Survey 2020 National Report for Health and Health and Social Care Partnerships Contents Ministerial Foreword for Everyone Matters Pulse Survey Report 2020 ...................... 4 Introduction ................................................................................................................. 5 Background .............................................................................................................. 5 The Pulse Survey ..................................................................................................... 6 Fieldwork .................................................................................................................. 6 Report ...................................................................................................................... 6 Staff Experience Stories .......................................................................................... 6 Response Rates Overview .......................................................................................... 7 Overall Response Rate ............................................................................................ 7 Reasons for Lower Response Rate ...................................................................... 8 Partials and Non-Completion ................................................................................ 8 Survey Methods ......................................................................................................