Pollen Types in the Epacridaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Harvest Significant Flora Surveys: Package 27-1 Prepared For: Vicforests
Pre-harvest Significant Flora Surveys: Package 27-1 Prepared for: VicForests 11 December 2018 Document Information Assessment Pre-harvest Significant Flora Surveys: Package 27-1 Project # 18-033 Prepared by Prepared for VicForests Distribution File Pre-harvest Flora Surveys_Package 27-1_Final_TactEcol_11Dec18 Document History Version Changes Author Date 21 November 2018 Draft v1 Draft report Address comments from 11 December 018 Final VicForests © This publication is copyright. It may only be used for the purposes for which it was commissioned and in accordance with any terms of engagement for the commission. The unauthorised use or reproduction in whole or in part of this publication is an infringement of copyright. Disclaimer: TactEcol Consulting Pty Ltd have taken all necessary steps to ensure this document is accurate and accords with relevant federal, state and local legislation, as well as industry best practice. The company accepts no liability for any damages or loss incurred as a result of reliance placed upon the report content or for any purpose other than that for which it was intended. 2 | Pre-harvest Significant Flora Surveys: Package 27-1 Table of Contents Document Information ...................................................................................................................................................... 2 1. Introduction ............................................................................................................................................................ 4 Study Area ........................................................................................................................................................................ -

Comparative Floral Presentation and Bee-Pollination in Two Sprengelia Species (Ericaceae)
Comparative floral presentation and bee-pollination in two Sprengelia species (Ericaceae) Karen A. Johnson* and Peter B. McQuillan School of Geography and Environmental Studies, University of Tasmania, Private Bag 78, Hobart, Tasmania 7001, Australia. *Corresponding author. E-mail: [email protected] Abstract: Pollination by sonication is unusual in the Styphelioideae, family Ericaceae. Sprengelia incarnata and Sprengelia propinqua have floral characteristics that suggested they might be adapted to buzz pollination.Both species have florally similar nectarless flowers except that the stamens ofSprengelia propinqua spread widely after the flower opens, while those of Sprengelia incarnata cohere in the centre of the flower. To test whether sonication occurs, we observed bee behaviour at the flowers of both plant species, documented potential pollinators, and examined their floral and pollen attributes. We found that Sprengelia incarnata had smaller and drier pollen than Sprengelia propinqua. We found that Sprengelia incarnata was sonicated by native bees in the families Apidae (Exoneura), Halictidae (Lasioglossum) and Colletidae (Leioproctus, Euryglossa). Sprengelia propinqua was also visited by bees from the Apidae (Exoneura) and Halictidae (Lasioglossum), but pollen was collected by scraping. The introduced Apis mellifera (Apidae) foraged at Sprengelia propinqua but ignored Sprengelia incarnata. The two Sprengelia species shared some genera of potential pollinators, but appeared to have diverged enough in their floral and pollen characters to elicit different behaviours from the native and introduced bees. Cunninghamia (2011) 12 (1): 45–51 Introduction species, some Leucopogon species, Richea milliganii (Hook.f.) F.Muell., and Sprengelia incarnata Sm. (Houston The interactions between plants and pollinators are thought & Ladd, 2002; Ladd, 2006). -

Vegetation Benchmarks Rainforest and Related Scrub
Vegetation Benchmarks Rainforest and related scrub Eucryphia lucida Vegetation Condition Benchmarks version 1 Rainforest and Related Scrub RPW Athrotaxis cupressoides open woodland: Sphagnum peatland facies Community Description: Athrotaxis cupressoides (5–8 m) forms small woodland patches or appears as copses and scattered small trees. On the Central Plateau (and other dolerite areas such as Mount Field), broad poorly– drained valleys and small glacial depressions may contain scattered A. cupressoides trees and copses over Sphagnum cristatum bogs. In the treeless gaps, Sphagnum cristatum is usually overgrown by a combination of any of Richea scoparia, R. gunnii, Baloskion australe, Epacris gunnii and Gleichenia alpina. This is one of three benchmarks available for assessing the condition of RPW. This is the appropriate benchmark to use in assessing the condition of the Sphagnum facies of the listed Athrotaxis cupressoides open woodland community (Schedule 3A, Nature Conservation Act 2002). Benchmarks: Length Component Cover % Height (m) DBH (cm) #/ha (m)/0.1 ha Canopy 10% - - - Large Trees - 6 20 5 Organic Litter 10% - Logs ≥ 10 - 2 Large Logs ≥ 10 Recruitment Continuous Understorey Life Forms LF code # Spp Cover % Immature tree IT 1 1 Medium shrub/small shrub S 3 30 Medium sedge/rush/sagg/lily MSR 2 10 Ground fern GF 1 1 Mosses and Lichens ML 1 70 Total 5 8 Last reviewed – 2 November 2016 Tasmanian Vegetation Monitoring and Mapping Program Department of Primary Industries, Parks, Water and Environment http://www.dpipwe.tas.gov.au/tasveg RPW Athrotaxis cupressoides open woodland: Sphagnum facies Species lists: Canopy Tree Species Common Name Notes Athrotaxis cupressoides pencil pine Present as a sparse canopy Typical Understorey Species * Common Name LF Code Epacris gunnii coral heath S Richea scoparia scoparia S Richea gunnii bog candleheath S Astelia alpina pineapple grass MSR Baloskion australe southern cordrush MSR Gleichenia alpina dwarf coralfern GF Sphagnum cristatum sphagnum ML *This list is provided as a guide only. -

Edition 2 from Forest to Fjaeldmark the Vegetation Communities Highland Treeless Vegetation
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Highland treeless vegetation Richea scoparia Edition 2 From Forest to Fjaeldmark 1 Highland treeless vegetation Community (Code) Page Alpine coniferous heathland (HCH) 4 Cushion moorland (HCM) 6 Eastern alpine heathland (HHE) 8 Eastern alpine sedgeland (HSE) 10 Eastern alpine vegetation (undifferentiated) (HUE) 12 Western alpine heathland (HHW) 13 Western alpine sedgeland/herbland (HSW) 15 General description Rainforest and related scrub, Dry eucalypt forest and woodland, Scrub, heathland and coastal complexes. Highland treeless vegetation communities occur Likewise, some non-forest communities with wide within the alpine zone where the growth of trees is environmental amplitudes, such as wetlands, may be impeded by climatic factors. The altitude above found in alpine areas. which trees cannot survive varies between approximately 700 m in the south-west to over The boundaries between alpine vegetation communities are usually well defined, but 1 400 m in the north-east highlands; its exact location depends on a number of factors. In many communities may occur in a tight mosaic. In these parts of Tasmania the boundary is not well defined. situations, mapping community boundaries at Sometimes tree lines are inverted due to exposure 1:25 000 may not be feasible. This is particularly the or frost hollows. problem in the eastern highlands; the class Eastern alpine vegetation (undifferentiated) (HUE) is used in There are seven specific highland heathland, those areas where remote sensing does not provide sedgeland and moorland mapping communities, sufficient resolution. including one undifferentiated class. Other highland treeless vegetation such as grasslands, herbfields, A minor revision in 2017 added information on the grassy sedgelands and wetlands are described in occurrence of peatland pool complexes, and other sections. -
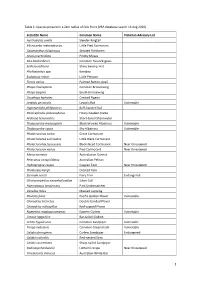
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Flora and Vegetation Survey of the Proposed Kwinana to Australind Gas
__________________________________________________________________________________ FLORA AND VEGETATION SURVEY OF THE PROPOSED KWINANA TO AUSTRALIND GAS PIPELINE INFRASTRUCTURE CORRIDOR Prepared for: Bowman Bishaw Gorham and Department of Mineral and Petroleum Resources Prepared by: Mattiske Consulting Pty Ltd November 2003 MATTISKE CONSULTING PTY LTD DRD0301/039/03 __________________________________________________________________________________ TABLE OF CONTENTS Page 1. SUMMARY............................................................................................................................................... 1 2. INTRODUCTION ..................................................................................................................................... 2 2.1 Location................................................................................................................................................. 2 2.2 Climate .................................................................................................................................................. 2 2.3 Vegetation.............................................................................................................................................. 3 2.4 Declared Rare and Priority Flora......................................................................................................... 3 2.5 Local and Regional Significance........................................................................................................... 5 2.6 Threatened -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Pollination Ecology and Evolution of Epacrids
Pollination Ecology and Evolution of Epacrids by Karen A. Johnson BSc (Hons) Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania February 2012 ii Declaration of originality This thesis contains no material which has been accepted for the award of any other degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Karen A. Johnson Statement of authority of access This thesis may be made available for copying. Copying of any part of this thesis is prohibited for two years from the date this statement was signed; after that time limited copying is permitted in accordance with the Copyright Act 1968. Karen A. Johnson iii iv Abstract Relationships between plants and their pollinators are thought to have played a major role in the morphological diversification of angiosperms. The epacrids (subfamily Styphelioideae) comprise more than 550 species of woody plants ranging from small prostrate shrubs to temperate rainforest emergents. Their range extends from SE Asia through Oceania to Tierra del Fuego with their highest diversity in Australia. The overall aim of the thesis is to determine the relationships between epacrid floral features and potential pollinators, and assess the evolutionary status of any pollination syndromes. The main hypotheses were that flower characteristics relate to pollinators in predictable ways; and that there is convergent evolution in the development of pollination syndromes. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -

Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: a Critical Review and Data Analysis
Environmental Science: Nano Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis Journal: Environmental Science: Nano Manuscript ID EN-CRV-04-2019-000461.R1 Article Type: Critical Review Date Submitted by the 10-Jun-2019 Author: Complete List of Authors: Jassby, David; University of California Los Angeles, Civil and Environmental Engineering Su, Yiming; Tongji Univ, Environmental Science and Technology; University of California, Los Angeles, Civil and Environmental Engineering Kim, Caroline; University of California Los Angeles, Civil and Environmental Engineering Ashworth, Vanessa; University of California Riverside Adeleye, Adeyemi; University of California Irvine Henry Samueli School of Engineering, Civil & Environmental Engineering Rolshausen, Philippe; University of California Riverside Roper, Caroline; University of California Riverside White, Jason; CT Agricultural Experiment Station, Analytical Chemistry Page 1 of 45 Environmental Science: Nano 1 2 3 Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical 4 5 Review and Data Analysis 6 7 Yiming Su1, Vanessa Ashworth2, Caroline Kim1, Adeyemi S. Adeleye3, Philippe Rolshausen2, 8 9 Caroline Roper4, Jason White5, David Jassby1,* 10 1Department of Civil and Environmental Engineering, University of California, Los Angeles 11 2 12 Department of Botany and Plant Sciences, University of California, Riverside 13 31Department of Civil and Environmental Engineering, University of California, Irvine 14 4Department of Plant Pathology, University of California, Riverside 15 5 16 Department of Analytical Chemistry, The Connecticut Agricultural Experiment Station 17 18 *Correspondence to: University of California, Los Angeles, Los Angeles, CA 90095, USA 19 20 Tel: 310-825-1346 21 Email: [email protected] 22 23 24 25 Abstract: 26 The increasing demand for food coupled to various environmental pressures, is increasing the 27 28 importance of sustainable agricultural practices. -

South West Region
Regional Services Division – South West Region South West Region ‐ Parks & Wildlife and FPC Disturbance Operations Flora and Vegetation Survey Assessment Form 1. Proposed Operations: (to be completed by proponent) NBX0217 Summary of Proposed Operation: Road Construction and Timber Harvesting New road construction – 3.75km Existing road upgrade – 14.9km New gravel pit construction – 2ha (exploration area) Contact Person and Contact Details: Adam Powell [email protected] 0427 191 332 Area of impact; District/Region, State Forest Block, Coupe/Compartment (shapefile to be provided): Blackwood District South West Region Barrabup 0317 Period of proposed disturbance: November 2016 to December 2017 1 2.Desktop Assessment: (to be completed by the Region) ‐ Check Forest Ecosystem reservation. Forest Ecosystems proposed for impact: Jarrah Forest‐Blackwood Plateau, Shrub, herb and sedgelands, Darling Scarp Y Are activities in a Forest Ecosystem that triggers informal reservation under the FMP? The Darling Scarp Forest Ecosystem is a Poorly Reserved Forest Ecosystem and needs to be protected as an Informal Reserve under the Forest Management Plan (Appendix 11) ‐ Check Vegetation Complexes, extents remaining uncleared and in reservation (DEC 2007/EPA 2006). Vegetation Complex Pre‐European extent (%) Pre‐European extent (Ha) Extent in formal/informal reservation (%) Bidella (BD) 94% 44,898 47% Darling Scarp (DS) Figures not available Corresponds to Darling Scarp Forest Ecosystem extent Gale (GA) 80% 899 17% Jalbarragup (JL) 91% 14,786 32% Kingia (KI) 96% 97,735 34% Telerah (TL) 92% 25,548 33% Wishart (WS2) 84% 2,796 35% Y Do any complexes trigger informal reservation under the FMP? Darling Scarp complex as discussed above Y Are any complexes significant as per EPA regionally significant vegetation? Gale (GA) complex is cleared below the recommended retention of 1,500ha (Molloy et.al 2007) ‐ Check Threatened flora and TEC/PEC databases over an appropriate radius of the disturbance boundary. -

Appendix F- H (PDF, 4.64
APPENDIX F Analysis to Investigate TEC SCP20a Presence Perth–Darwin National Highway (Swan Valley Section)– Supplementary Biological Studies 2015 Assessment of the Presence of the TEC SCP20a at Ioppolo Rd, Chittering COFFEY NOVEMBER 2015 TEL. (08) 9315 4688 [email protected] PO Box 50, Applecross WA 6953 www.woodmanenv.com.au Coffey Perth–Darwin National Highway (Swan Valley Section)– Supplementary Biological Studies 2015 Assessment of the presence of the TEC SCP20a at Ioppolo Rd, Chittering Perth–Darwin National Highway (Swan Valley Section) – Supplementary Biological Studies 2015: Assessment of the presence of the TEC SCP20a at Ioppolo Rd, Chittering Prepared for: Coffey Job Number: Coffey15‐28 Report Number: Coffey15‐28‐03 Cover Photograph: Lot M2019 ‐ Ioppolo Road, waypoint 82 (Woodman Environmental) DOCUMENT REVISION AND STATUS Revision Status Originator Internal Internal Client Client Reviewer Review Date Reviewer Review Date A Draft report BL CG/GW 22/10/2015 N. Raymond 16/11/2015 Main Roads B Client Comments BL GW 19/11/2015 D. Morley 26/11/2015 Incorporated Main Roads 0 Final report BL GW 26/11/2015 DISCLAIMER This document is prepared in accordance with and subject to an agreement between Woodman Environmental Consulting Pty Ltd (“Woodman Environmental”) and the client for whom it has been prepared (“Coffey”) and is restricted to those issues that have been raised by the Client in its engagement of Woodman Environmental and prepared eusing th standard of skill and care ordinarily exercised by Environmental Scientists