Population Genetics and Distribution of Two Sympatric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibian Abundance and Detection Trends During a Large Flood in a Semi-Arid Floodplain Wetland
Herpetological Conservation and Biology 11:408–425. Submitted: 26 January 2016; Accepted: 2 September 2016; Published: 16 December 2016. Amphibian Abundance and Detection Trends During a Large Flood in a Semi-Arid Floodplain Wetland Joanne F. Ocock1,4, Richard T. Kingsford1, Trent D. Penman2, and Jodi J.L. Rowley1,3 1Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, UNSW Australia, Sydney, New South Wales, 2052, Australia 2Centre for Environmental Risk Management of Bushfires, Institute of Conservation Biology and Environmental Management, University of Wollongong, Wollongong, New South Wales 2522, Australia 3Australian Museum Research Institute, Australian Museum, 6 College St, Sydney, New South Wales 2010, Australia 4Corresponding author, email: [email protected] Abstract.—Amphibian abundance and occupancy are often reduced in regulated river systems near dams, but com- paratively little is known about how they are affected on floodplain wetlands downstream or the effects of actively managed flows. We assessed frog diversity in the Macquarie Marshes, a semi-arid floodplain wetland of conserva- tion significance, identifying environmental variables that might explain abundances and detection of species. We collected relative abundance data of 15 amphibian species at 30 sites over four months, coinciding with a large natural flood. We observed an average of 39.9 ± (SE) 4.3 (range, 0-246) individuals per site survey, over 47 survey nights. Three non-burrowing, ground-dwelling species were most abundant at temporarily flooded sites with low- growing aquatic vegetation (e.g., Limnodynastes tasmaniensis, Limnodynastes fletcheri, Crinia parinsignifera). Most arboreal species (e.g., Litoria caerulea) were more abundant in wooded habitat, regardless of water permanency. -

Threat Abatement Plan
gus resulting in ch fun ytridio trid myc chy osis ith w s n ia ib h p m a f o n o i t THREAT ABATEMENTc PLAN e f n I THREAT ABATEMENT PLAN INFECTION OF AMPHIBIANS WITH CHYTRID FUNGUS RESULTING IN CHYTRIDIOMYCOSIS Department of the Environment and Heritage © Commonwealth of Australia 2006 ISBN 0 642 55029 8 Published 2006 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth, available from the Department of the Environment and Heritage. Requests and inquiries concerning reproduction and rights should be addressed to: Assistant Secretary Natural Resource Management Policy Branch Department of the Environment and Heritage PO Box 787 CANBERRA ACT 2601 This publication is available on the Internet at: www.deh.gov.au/biodiversity/threatened/publications/tap/chytrid/ For additional hard copies, please contact the Department of the Environment and Heritage, Community Information Unit on 1800 803 772. Front cover photo: Litoria genimaculata (Green-eyed tree frog) Sequential page photo: Taudactylus eungellensis (Eungella day frog) Banner photo on chapter pages: Close up of the skin of Litoria genimaculata (Green-eyed tree frog) ii Foreword ‘Infection of amphibians with chytrid fungus resulting Under the EPBC Act the Australian Government in chytridiomycosis’ was listed in July 2002 as a key implements the plan in Commonwealth areas and seeks threatening process under the Environment Protection the cooperation of the states and territories where the and Biodiversity Conservation Act 1999 (EPBC Act). disease impacts within their jurisdictions. -

Expert Witness Report
Expert Witness Report Report prepared on instructions of: Bleyer Lawyers, Level 1, 550 Lonsdale Street, Melbourne, Vic 3000 Australia Prepared by: Graeme Gillespie B.Sc. Ph.D. 55 Union Street, Northcote, Vic 3070, Australia Curriculum Vitae Attached (Appendix I) I have read the Expert Witness Code of Conduct and agree to be bound by it. Graeme Gillespie 23 February 2010 Qualifications and Experience Please see my curriculum vitae (Appendix I) for my general qualifications and experience. My Ph.D. in zoology focussed specifically on the conservation biology and ecology of frog species in south-eastern Australia. I have 23 years of field and scientific experience studying amphibians and their conservation and management in south- eastern Australia. I have published 24 refereed scientific papers and 38 technical reports on amphibian ecology, conservation and management. I am recognised throughout Australia as an authority on the frog fauna of Victoria, specifically with respect to conservation issues, and I am regularly asked to provide advice on such matters to individuals, government conservation and land management agencies, and non-government organisations. With regard to the Giant Burrowing Frog, I encountered this species on several occasions between 1986 and 1992 while undertaking and supervising pre-logging biodiversity surveys in East Gippsland, Victoria. These records are documented in the Victorian Wildlife Atlas. During this period, I gained knowledge of the species’ habitat associations, breeding biology, some aspects of its behaviour and an appreciation of its conservation status in Victoria (see Opie et al. 1990; Westaway et al.1990; Lobert et al. 1991). Because of my research into amphibian conservation and management, I am highly familiar with the existing literature on the impact of various forest management activities on amphibians and the implications of these activities for amphibian conservation. -

Oleon Palm Mill List 2019 Short.Xlsx
Oleon NV palm mill list 2019 version 06/07/2020 # Mill name Mill parent company Country Location Latitude Longitude 1 AATHI BAGAWATHI MANUFACTUR ABDI BUDI MULIA Indonesia NORTH SUMATRA 2.05228 100.25207 2 ABAGO S.A.S. PALMICULTORES DEL NORTE Colombia Km 17 vía Dinamarca, Acacías - Meta 3.960839 -73.627319 3 ABDI BUDI MULIA 1 SUMBER TANI HARAPAN (STH) Indonesia NORTH SUMATRA 2.05127 100.25234 4 ABDI BUDI MULIA 2 SUMBER TANI HARAPAN (STH) Indonesia NORTH SUMATRA 2.11272 100.27311 5 Abedon Oil Mill Kretam Holdings Bhd Malaysia 56KM, Jalan Lahad DatuSandakan, 90200 Kinabatangan, Sabah 5.312372 117.978891 6 ACE OIL MILL S/B ACE OIL MILL SDN. BHD Malaysia KM22, Lebuhraya Keratong-Bahau, Rompin, Pahang 2.91192 102.77981 7 Aceites Cimarrones S.A.S. Aceites Cimarrones S.A.S. Colombia Fca Tucson II Vda Candelejas, Puerto Rico, Meta 3.03559 -73.11147 8 ACEITES S.A. ACEITES S.A. Colombia MAGDALENA 10.56788889 -74.20816667 9 Aceites Y Derivados S.A. Aceites Y Derivados S.A. Honduras KM 348, Carretera Al Batallon Xatruch, Aldea Los Leones, Trujillo, Colon 15.825861 -85.896861 10 ACEITES Y GRASAS DEL CATATUMBO SAS OLEOFLORES S.A. Colombia META 3.718639 -73.701775 11 ACHIJAYA ACHIJAYA PLANTATION Malaysia Lot 677, Jalan Factory, Chaah, Johor 85400 2.204167 103.041389 12 Adela FGV PALM INDUSTRIES SDN BHD Malaysia Adela, 81930 Bandar Penawar, Johor Darul Takzim 1.551917 104.186361 13 ADHIRADJA CHANDRA BUANA ADHIRADJA CHANDRA BUANA Indonesia JAMBI -1.6797 103.80176 14 ADHYAKSA DHARMA SATYA EAGLE HIGH PLANTATIONS Indonesia CENTRAL KALIMANTAN -1.58893 112.86188 15 Adimulia Agrolestari ADIMULIA AGRO LESTARI Indonesia Subarak, Gn. -

FREE Penang Traveller's
HOMESTAY FOREIGN MISSIONS Bus Information Explore Penang by Rapid Penang Chingay - A National Tourism Malaysia, Northern Region 04-261 9067 Austria 04-656 8525 Bangladesh 04-262 1085 Cultural Heritage From Komtar Bus Terminal to: Bus Number(s): INTERNATIONAL SOCIETIES Britain 04-227 5336 • Kapitan Keling Mosque 301 / 302 / 303 / 401 Alliance Francaise 04-227 6008 Canada 04-389 3300 • Kek Lok Si Temple 201 / 203 / 204 / 502 Denmark 04-262 4886 32 British Council 04-263 0330 • Little India 101 / 104 / 201 / 202 / 203 Malaysian German Society 04-226 0734 Finland 04-229 4300 UNESCO WORLD CULTURAL HERITAGE CITY • Wat Chayamangkalaram 101 / 103 / 104 The Penang Japanese Association 04-229 3257 France 04-642 2611 • Khoo Kongsi 301 / 302 / T10 / 401 YMCA 04-228 8211 Germany 04-647 1288 YWCA 04-828 1855 Hungary 04-644 9937 • Snake Temple 401 / 401E Indonesia 04-227 4686 • War Museum 302 / 307 LIBRARIES Japan 04-226 3030 • Museum & Art Gallery CAT buses / 103 / 204 / 502 Netherlands 04-647 3310 THE HISTORIC CITY OF GEORGE TOWN • P. Ramlee’s House 206 Alliance Francaise French Library 04-227 6008 Norway 04-226 3905 Sunday & Monday: Close Pakistan 04-282 9800 On 7th July 2008, George Town was awarded UNESCO World Heritage status. Founded • Fort Cornwallis CAT buses / 103 / 204 / 502 Malaysian German Society 04-226 0734 • Toy Museum 101 / 103 / 104 Russia 04-229 0127 Penang Chinese Town Hall Library 04-262 8939 Sweden & Norway 04-226 3459 200 years ago, the city has an impressive collection of historic buildings representing • Cheong Fatt Tze Mansion 103 / 204 / T10 Penang Georgetown Library 04-229 3555 Thailand 04-226 8029 the cultural heritage of Penang’s various ethnicities: Chinese, Indians, Arabs, Malays, • Forestry Museum 101 Wednesday-Sunday: 9.45am – 6.00pm JL N. -

The Provider-Based Evaluation (Probe) 2014 Preliminary Report
The Provider-Based Evaluation (ProBE) 2014 Preliminary Report I. Background of ProBE 2014 The Provider-Based Evaluation (ProBE), continuation of the formerly known Malaysia Government Portals and Websites Assessment (MGPWA), has been concluded for the assessment year of 2014. As mandated by the Government of Malaysia via the Flagship Coordination Committee (FCC) Meeting chaired by the Secretary General of Malaysia, MDeC hereby announces the result of ProBE 2014. Effective Date and Implementation The assessment year for ProBE 2014 has commenced on the 1 st of July 2014 following the announcement of the criteria and its methodology to all agencies. A total of 1086 Government websites from twenty four Ministries and thirteen states were identified for assessment. Methodology In line with the continuous and heightened effort from the Government to enhance delivery of services to the citizens, significant advancements were introduced to the criteria and methodology of assessment for ProBE 2014 exercise. The year 2014 spearheaded the introduction and implementation of self-assessment methodology where all agencies were required to assess their own websites based on the prescribed ProBE criteria. The key features of the methodology are as follows: ● Agencies are required to conduct assessment of their respective websites throughout the year; ● Parents agencies played a vital role in monitoring as well as approving their agencies to be able to conduct the self-assessment; ● During the self-assessment process, each agency is required to record -

Spatial Ecology of the Giant Burrowing Frog (Heleioporus Australiacus): Implications for Conservation Prescriptions
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health January 2008 Spatial ecology of the giant burrowing frog (Heleioporus australiacus): implications for conservation prescriptions Trent D. Penman University of Wollongong, [email protected] F Lemckert M J Mahony Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Penman, Trent D.; Lemckert, F; and Mahony, M J: Spatial ecology of the giant burrowing frog (Heleioporus australiacus): implications for conservation prescriptions 2008, 179-186. https://ro.uow.edu.au/scipapers/724 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Spatial ecology of the giant burrowing frog (Heleioporus australiacus): implications for conservation prescriptions Abstract Management of threatened anurans requires an understanding of a species’ behaviour and habitat requirements in both the breeding and non-breeding environments. The giant burrowing frog (Heleioporus australiacus) is a threatened species in south-eastern Australia. Little is known about its habitat requirements, creating difficulties in vde eloping management strategies for the species.Weradio-tracked 33 individual H. australiacus in order to determine their habitat use and behaviour. Data from 33 frogs followed for between 5 and 599 days show that individuals spend little time near (<15 >m) their breeding sites (mean 4.7 days for males and 6.3 days for females annually). Most time is spent in distinct non- breeding activity areas 20–250m from the breeding sites. -

Thermal Surface Analysis on Neo-Minimalist Apartment Façades in Penang, Malaysia
SHS Web of Conferences 45, 01001 (2018) https://doi.org/10.1051/shsconf/20184501001 ICLK 2017 Thermal Surface Analysis on Neo-minimalist apartment façades in Penang, Malaysia Yasser Arab1,1, Ahmad Sanusi Hassan1, and Bushra Qanaa2 1School of Housing, Building, and Planning. Universiti Sains Malaysia 2Faculty of Architecture, Ittihad Private University Abstract. The study investigates apartment’s façade thermal performance with neo-minimalist architectural style in Penang, Malaysia. Neo-minimalist style is considered as the most popular style in Malaysia in 2010s. The style is rediscovering from early modern minimalist movement with a design concept “less is more”. It applies minimal and efficient design of architectural character in defining form and space. Penang Island the second most important city in Malaysia after Kuala Lumpur. It is located at the north-western part of the country. The first case studies is the Light Linear apartment which has sixteen stories located on the east cost of Penang Island at Pantai Street, Penang. The second case study is BayStar apartment building, the eleven stories building tis located in Bayan Lepas at the seaside facing Jerejak Island. In order to conduct this study Fluke Ti20 thermal imager was used to capture thermal images for the west facades of the selected case study hourly from 12:00 to 6:00 pm on 15th March 2017. The study finds that the recessed wall, balconies and the shading devices were the important elements to provide shades on the façades for good thermal performance. 1 INTRODUCTION The study aims to investigate the façade surface temperature of two neo-minimalist architecture style high- rise buildings within Penang Island, Malaysia. -

National Recovery Plan for the Stuttering Frog Mixophyes Balbus
National Recovery Plan for the Stuttering Frog Mixophyes balbus David Hunter and Graeme Gillespie Prepared by David Hunter and Graeme Gillespie (Department of Sustainability and Environment, Victoria). Published by the Victorian Government Department of Sustainability and Environment (DSE) Melbourne, October 2011. © State of Victoria Department of Sustainability and Environment 2010 This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968. Authorised by the Victorian Government, 8 Nicholson Street, East Melbourne. ISBN 978-1-74242-369-2 (online) This is a Recovery Plan prepared under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999, with the assistance of funding provided by the Australian Government. This Recovery Plan has been developed with the involvement and cooperation of a range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. Disclaimer: This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence that may arise from you relying on any information in this publication. An electronic version of this document is available on the Department of the Environment, Water, Heritage and the Arts website www.environment.gov.au For more information contact the DSE Customer Service Centre 136 186 Citation: Hunter, D. -
X X X X L L L L L L L X L L L L L L L L L X L L X L L X X X X
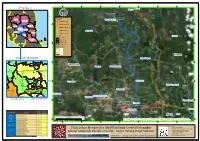
-24000 -18000 -12000 -6000 0 6000 12000 J J a a l l a Burau a Pelan Kunci n n Burau P P e e r r s s e Kelantan e k k u Kg Batu Gading u tu an t a u u kut n Kuala KraJ u erse a a n P n la Jala Terengganu Z Xn N e g e Tmn Setia r Lipis Petunjuk Kuala Kerau i Kuala Krau Jerantut X l KLUSTER PAYA PULAI Tmn Sri Desa X Perak 0 0 3 0 2 0 0 1 0 2 SARINGAN BERGEJALA C 2 - - n i X r tua Kuantan ku J e Raub rse a g e la e Pahang SARINGAN KONTAK RAPAT P n n N X ala N J eg n e a Temerloh ri l Maran C a SARINGAN KONTAK 1 J X Jenderak 23 J Bentong a Pekan la SARINGAN SUKARELA n Selangor X N e Kuala Lumpur Bera g e Jalan r i Chenor C Sungai Utama 1 3 9 Sempadan Negeri Negeri Sembilan Rompin Sempadan Daerah 0 0 0 Sempadan Mukim 0 0 0 8 8 - Sempadan Temerloh J - ala Melaka Johor n Ut am Status Lokaliti a J en gk a 8 ,9 ,1 2, 13 Maran Daerah Temerloh Lipat Kajang 0 0 0 0 0 0 0Dong Ulu Cheka 4 4 0 0 Pulau Tawar0 1 1 0Ulu Dong 0 - - Tebing Tinggi Kelola0 an Negeri C137 Burau Temerloh Jal Jerantut0 Kerdau Pekan Kerdau 9 Raub Gali Jenderak 10 Chenor 00 0 4 Maran 6 Pahang r Temerloh Lipat Kajang 0 u 0 0 0 im 0 0 0 T Bentong Kerdau 0 0 Sanggang ai 0 0 2 t 2 35 57 - Semantan an - J P a a Semantan l Sanggang0 a ay 1 n hr Jalan Ne u N geri C1 Bukit Segumpal 0 0Songsang J 21 b e e 0 a L g J l Mentakab a a 4 1 e la n n 1Bangau r i N e P C ta s e Temerloh 0 1 1 r g M 2 a s J n Songsang 6 21 ju e J a a a k Sabai l l d a 0 u a 0 a n n Perak t 40 u L C Lebak P 0 a h 0 n Bentong n e e a Kg Baru Mentakab k n Bera l 9 o Lanchang a a 8 r n 3 J n J 2 Jala a 57 0 l S 1 8 X -

(CPRC), Disease Control Division, the State Health Departments and Rapid Assessment Team (RAT) Representative of the District Health Offices
‘Annex 26’ Contact Details of the National Crisis Preparedness & Response Centre (CPRC), Disease Control Division, the State Health Departments and Rapid Assessment Team (RAT) Representative of the District Health Offices National Crisis Preparedness and Response Centre (CPRC) Disease Control Division Ministry of Health Malaysia Level 6, Block E10, Complex E 62590 WP Putrajaya Fax No.: 03-8881 0400 / 0500 Telephone No. (Office Hours): 03-8881 0300 Telephone No. (After Office Hours): 013-6699 700 E-mail: [email protected] (Cc: [email protected] and [email protected]) NO. STATE 1. PERLIS The State CDC Officer Perlis State Health Department Lot 217, Mukim Utan Aji Jalan Raja Syed Alwi 01000 Kangar Perlis Telephone: +604-9773 346 Fax: +604-977 3345 E-mail: [email protected] RAT Representative of the Kangar District Health Office: Dr. Zulhizzam bin Haji Abdullah (Mobile: +6019-4441 070) 2. KEDAH The State CDC Officer Kedah State Health Department Simpang Kuala Jalan Kuala Kedah 05400 Alor Setar Kedah Telephone: +604-7741 170 Fax: +604-7742 381 E-mail: [email protected] RAT Representative of the Kota Setar District Health Office: Dr. Aishah bt. Jusoh (Mobile: +6013-4160 213) RAT Representative of the Kuala Muda District Health Office: Dr. Suziana bt. Redzuan (Mobile: +6012-4108 545) RAT Representative of the Kubang Pasu District Health Office: Dr. Azlina bt. Azlan (Mobile: +6013-5238 603) RAT Representative of the Kulim District Health Office: Dr. Sharifah Hildah Shahab (Mobile: +6019-4517 969) 71 RAT Representative of the Yan District Health Office: Dr. Syed Mustaffa Al-Junid bin Syed Harun (Mobile: +6017-6920881) RAT Representative of the Sik District Health Office: Dr. -

KKM HEADQUARTERS Division / Unit Activation Code PEJABAT Y.B. MENTERI 3101010001 PEJABAT Y.B
KKM HEADQUARTERS Division / Unit Activation Code PEJABAT Y.B. MENTERI 3101010001 PEJABAT Y.B. TIMBALAN MENTERI 3101010002 PEJABAT KETUA SETIAUSAHA 3101010003 PEJABAT TIMBALAN KETUA SETIAUSAHA (PENGURUSAN) 3101010004 PEJABAT TIMBALAN KETUA SETIAUSAHA (KEWANGAN) 3101010005 PEJABAT KETUA PENGARAH KESIHATAN 3101010006 PEJABAT TIMBALAN KETUA PENGARAH KESIHATAN (PERUBATAN) 3101010007 PEJABAT TIMBALAN KETUA PENGARAH KESIHATAN (KESIHATAN AWAM) 3101010008 PEJABAT TIMBALAN KETUA PENGARAH KESIHATAN (PENYELIDIKAN DAN SOKONGAN TEKNIKAL) 3101010009 PEJABAT PENGARAH KANAN (KESIHATAN PERGIGIAN) 3101010010 PEJABAT PENGARAH KANAN (PERKHIDMATAN FARMASI) 3101010011 PEJABAT PENGARAH KANAN (KESELAMATAN DAN KUALITI MAKANAN) 3101010012 BAHAGIAN AKAUN 3101010028 BAHAGIAN AMALAN DAN PERKEMBANGAN FARMASI 3101010047 BAHAGIAN AMALAN DAN PERKEMBANGAN KESIHATAN PERGIGIAN 3101010042 BAHAGIAN AMALAN PERUBATAN 3101010036 BAHAGIAN DASAR DAN HUBUNGAN ANTARABANGSA 3101010019 BAHAGIAN DASAR DAN PERANCANGAN STRATEGIK FARMASI 3101010050 BAHAGIAN DASAR DAN PERANCANGAN STRATEGIK KESIHATAN PERGIGIAN 3101010043 BAHAGIAN DASAR PERANCANGAN STRATEGIK DAN STANDARD CODEX 3101010054 BAHAGIAN KAWALAN PENYAKIT 3101010030 BAHAGIAN KAWALAN PERALATAN PERUBATAN 3101010055 BAHAGIAN KAWALSELIA RADIASI PERUBATAN 3101010041 BAHAGIAN KEJURURAWATAN 3101010035 BAHAGIAN KEWANGAN 3101010026 BAHAGIAN KHIDMAT PENGURUSAN 3101010023 BAHAGIAN PEMAKANAN 3101010033 BAHAGIAN PEMATUHAN DAN PEMBANGUNAN INDUSTRI 3101010053 BAHAGIAN PEMBANGUNAN 3101010020 BAHAGIAN PEMBANGUNAN KESIHATAN KELUARGA 3101010029 BAHAGIAN