Heavy Halogen Geochemistry of Martian Shergottite Meteorites and 2 Implications for the Halogen Composition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
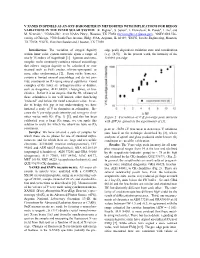
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

Petrography and Geochemistry of the Enriched Basaltic Shergottite Northwest Africa 2975
Meteoritics & Planetary Science 50, Nr 12, 2024–2044 (2015) doi: 10.1111/maps.12571 Petrography and geochemistry of the enriched basaltic shergottite Northwest Africa 2975 Qi HE1*, Long XIAO1, J. Brian BALTA2, Ioannis P. BAZIOTIS3, Weibiao HSU4, and Yunbin GUAN5 1Planetary Science Institute, School of Earth Sciences, China University of Geosciences, Wuhan 430074, China 2Department of Geology and Planetary Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania 15217, USA 3Agricultural University of Athens, Laboratory of Mineralogy and Geology, Athens 11855, Greece 4Laboratory for Astrochemistry and Planetary Sciences, Purple Mountain Observatory, Chinese Academy of Sciences, Nanjing 210008, China 5Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, USA *Corresponding author. E-mail: [email protected] (Received 30 July 2013; revision accepted 30 September 2015) Abstract–We present a study of the petrology and geochemistry of basaltic shergottite Northwest Africa 2975 (NWA 2975). NWA 2975 is a medium-grained basalt with subophitic to granular texture. Electron microprobe (EMP) analyses show two distinct pyroxene compositional trends and patchy compositional zoning patterns distinct from those observed in other meteorites such as Shergotty or QUE 94201. As no bulk sample was available to us for whole rock measurements, we characterized the fusion crust and its variability by secondary ion mass spectrometer (SIMS) measurements and laser ablation inductively coupled plasma spectroscopy (LA-ICP-MS) analyses as a best-available proxy for the bulk rock composition. The fusion crust major element composition is comparable to the bulk composition of other enriched basaltic shergottites, placing NWA 2975 within that sample group. The CI-normalized REE (rare earth element) patterns are flat and also parallel to those of other enriched basaltic shergottites. -

The Tissint Martian Meteorite As Evidence for the Largest Impact Excavation
ARTICLE Received 17 Jul 2012 | Accepted 20 Dec 2012 | Published 29 Jan 2013 DOI: 10.1038/ncomms2414 The Tissint Martian meteorite as evidence for the largest impact excavation Ioannis P. Baziotis1, Yang Liu1,2, Paul S. DeCarli3, H. Jay Melosh4, Harry Y. McSween1, Robert J. Bodnar5 & Lawrence A. Taylor1 High-pressure minerals in meteorites provide clues for the impact processes that excavated, launched and delivered these samples to Earth. Most Martian meteorites are suggested to have been excavated from 3 to 7 km diameter impact craters. Here we show that the Tissint meteorite, a 2011 meteorite fall, contains virtually all the high-pressure phases (seven minerals and two mineral glasses) that have been reported in isolated occurrences in other Martian meteorites. Particularly, one ringwoodite (75 Â 140 mm2) represents the largest grain observed in all Martian samples. Collectively, the ubiquitous high-pressure minerals of unusually large sizes in Tissint indicate that shock metamorphism was widely dispersed in this sample (B25 GPa and B2,000 1C). Using the size and growth kinetics of the ring- woodite grains, we infer an initial impact crater with B90 km diameter, with a factor of 2 uncertainty. These energetic conditions imply alteration of any possible low-T minerals in Tissint. 1 Planetary Geosciences Institute, Department of Earth and Planetary Sciences, University of Tennessee, Knoxville, Tennessee 37996, USA. 2 Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109, USA. 3 Poulter Laboratory, SRI International, Menlo Park, California 94025, USA. 4 Department of Earth, Atmospheric and Planetary Sciences, Purdue University, West Lafayette, Indiana 47907, USA. 5 Department of Geosciences, Virginia Tech, Blacksburg, Virginia 24061, USA. -

Meteorites: an Overview
Meteorites: An Overview Edward R. D. Scott* 1811-5209/11/0007-0047$2.50 DOI: 10.2113/gselements.7.1.47 eteorites come from numerous parent bodies with a wide variety chondrites are the most pristine, of geological histories. A few (~0.5%) come from Mars or the Moon; type 6 are the most metamor- phosed, and type 1 are the most Mthe rest are impact debris from collisions between asteroids orbiting aqueously altered. between Mars and Jupiter. Unlike terrestrial, Martian, and lunar rocks, the Asteroids that melted supply us asteroidal meteorites contain minerals that formed before the Sun and the with three major classes of mete- Solar System, during the growth of planetesimals and planets from the disk orites: irons, which are Fe–Ni of dust and gas around the Sun (“the solar nebula”), and during the first samples from the cores of aster- oids; metal-free silicate rocks from half-billion years of Solar System evolution. asteroidal mantles and crusts, Meteorites from unmelted asteroids are called chondrites; which are called achondrites as they are cosmic sediments composed of particles that were they lack chondrules; and stony irons, which are impact- generated mixtures of achondrites and Fe–Ni metal (Fig. present in the solar nebula (Fig. 1). The most abundant particles are millimeter-sized objects called chondrules, 2). Most irons (85%) are divided into thirteen groups which are solidified droplets of silicate magma. The chon- (called IAB, IIAB, IIC, etc.), which come from separate drules, associated grains of Fe–Ni metal, and a few volume parent bodies. The remaining ~15% probably come from percent or less of calcium–aluminum-rich inclusions another 50-odd parent bodies. -

Reviewing Martian Atmospheric Noble Gas Measurements: from Martian Meteorites to Mars Missions
geosciences Review Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions Thomas Smith 1,* , P. M. Ranjith 1, Huaiyu He 1,2,3,* and Rixiang Zhu 1,2,3 1 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, 19 Beitucheng Western Road, Box 9825, Beijing 100029, China; [email protected] (P.M.R.); [email protected] (R.Z.) 2 Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China 3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (T.S.); [email protected] (H.H.) Received: 10 September 2020; Accepted: 4 November 2020; Published: 6 November 2020 Abstract: Martian meteorites are the only samples from Mars available for extensive studies in laboratories on Earth. Among the various unresolved science questions, the question of the Martian atmospheric composition, distribution, and evolution over geological time still is of high concern for the scientific community. Recent successful space missions to Mars have particularly strengthened our understanding of the loss of the primary Martian atmosphere. Noble gases are commonly used in geochemistry and cosmochemistry as tools to better unravel the properties or exchange mechanisms associated with different isotopic reservoirs in the Earth or in different planetary bodies. The relatively low abundance and chemical inertness of noble gases enable their distributions and, consequently, transfer mechanisms to be determined. In this review, we first summarize the various in situ and laboratory techniques on Mars and in Martian meteorites, respectively, for measuring noble gas abundances and isotopic ratios. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Critically Testing Olivine-Hosted Putative Martian Biosignatures in the Yamato 000593
1 Critically testing olivine-hosted putative Martian biosignatures in the Yamato 000593 2 meteorite - geobiological implications 3 4 Abstract: 5 On rocky planets such as Earth and Mars the serpentinization of olivine in ultramafic crust 6 produces hydrogen that can act as a potential energy source for life. Direct evidence of fluid-rock 7 interaction on Mars comes from iddingsite alteration veins found in Martian meteorites. In the 8 Yamato 000593 meteorite putative biosignatures have been reported from altered olivines in the 9 form of microtextures and associated organic material that have been compared to tubular 10 bioalteration textures found in terrestrial sub-seafloor volcanic rocks. Here we use a suite of 11 correlative, high-sensitivity, in-situ chemical and morphological analyses to characterize and re- 12 evaluate these microalteration textures in Yamato 000593, a clinopyroxenite from the shallow sub- 13 surface of Mars. We show that the altered olivine crystals have angular and micro-brecciated 14 margins and are also highly strained due to impact induced fracturing. The shape of the olivine 15 microalteration textures is in no way comparable to microtunnels of inferred biological origin 16 found in terrestrial volcanic glasses and dunites, and rather we argue that the Yamato 000593 17 microtextures are abiotic in origin. Vein filling iddingsite extends into the olivine microalteration 18 textures and contains amorphous organic carbon occurring as bands and sub-spherical 19 concentrations <300 nm across. We propose that a Martian impact event produced the micro- 20 brecciated olivine crystal margins that reacted with subsurface hydrothermal fluids to form 21 iddingsite containing organic carbon derived from abiotic sources. -

Carbonaceous Particles in Rock of the Tissint Martian Meteorite
EPSC Abstracts Vol. 7 EPSC2012-906 2012 European Planetary Science Congress 2012 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2012 Carbonaceous particles in rock of the Tissint martian meteorite N. Miyake (1), M.K. Wallis (1,2), J. Wallis (3), S. Al-Mufti (1) and N.C. Wickramsinghe (1,2) 1 Buckingham Centre for Astrobiology, University of Buckingham, Buckingham MK18 1EG, UK 2 Cardiff University, 49b Park Place, Cardiff CF10 3AT, UK 3 School of Mathematics, Cardiff University, Cardiff, UK Abstract 2. Present Study Carbon-rich globules and plates sized 10-50µm in the Our sample of Tissint showed no fusion crust [2] Tissint martian meteorite lie within the fragile rock, from atmospheric friction, implying it was an interior made up of loosely consolidated micro-fragments. It fragment. We broke it up (clean handling in laminar is interpreted as wind-blown martian dust with rather flow cabinet) to find fresh interior surfaces for study. few carbonaceous spheroids that became buried in We found several 10-50µm globules and plates in the regolith until the impact ejection event. SEM images, embedded in the porous rocky matrix 1. Introduction to various extents, which EDAX spectra showed to The Tissint meteorite is one of the few meteorites be carbon/oxygen-rich. The 10µm egg-shaped observed on arrival in July 2011 and pieces were globule in Fig. A was reported earlier [3] and is here picked up after 3 months in the Moroccan desert [0]. shown in the very rough substrate of scale 1-10µm Most of the 60 or so martian meteorites have been with a diagonal crack that shows bulk coherence. -

Lu-Hf and Sm-Nd AGES and SOURCE COMPOSITIONS for DEPLETED
Lu-Hf AND Sm-Nd AGES AND SOURCE COMPOSITIONS FOR DEPLETED SHERGOTTITE TISSINT ________________________________ A Thesis Presented to the Faculty of the Department of Earth and Atmospheric Sciences University of Houston ________________________________ In Partial Fulfillment of the Requirements for the Degree Master of Science ________________________________ By Therica Esther Grosshans December 2013 Lu-Hf AND Sm-Nd AGES AND SOURCE COMPOSITIONS FOR DEPLETED SHERGOTTITE TISSINT ___________________________________ Therica Esther Grosshans APPROVED: ___________________________________ Dr. Thomas Lapen, Advisor ___________________________________ Dr. Alan Brandon ___________________________________ Dr. Rasmus Andreasen ___________________________________ Dr. Kevin Righter, Johnson Space Center ___________________________________ Dr. Dan Wells, Dean College of Natural Sciences and Mathematics ii ACKNOWLEDGEMENTS First, I would like to thank my advisor, Dr. Tom Lapen, for the opportunity to work on such a great project. Thank you for your time, guidance, and sharing your knowledge with me through the last few years. Next, I would like to thank my committee members. Thank you to Dr. Alan Brandon and Dr. Kevin Righter for your support and assistance throughout the course of my work. Thank you to Dr. Rasmus Andreasen for all your assistance on the MC-ICP-MS and involvement with my research. All your help, especially at 4 a.m. when the machine would stop running, during my research is much appreciated. Thank you to Dr. Minako Righter, Barry Shaulis, and Jesse Dietderich for their assistance with the LA-ICP-MS and spending a lot of your time helping me in the clean lab. We successfully made it through this research without an acid burn. However, some special glassware did not. A special thanks to my parents, Mike and Christie, and my sisters, Erin and Alicia, for their love and support while in school. -

Exploring Exogenic Sources for the Olivine on Asteroid (4) Vesta
Exploring Exogenic Sources for the Olivine on Asteroid (4) Vesta Lucille Le Corre Planetary Science Institute, 1700 East Fort Lowell, Suite 106, Tucson, AZ 85719, USA. Email: [email protected] Vishnu Reddy Planetary Science Institute, 1700 East Fort Lowell, Suite 106, Tucson, AZ 85719, USA. Juan A. Sanchez Planetary Science Institute, 1700 East Fort Lowell, Suite 106, Tucson, AZ 85719, USA. Tasha Dunn Colby College, Department of Geology, 5800 Mayflower Hill, Waterville, ME 04901 Edward A. Cloutis Department of Geography, University of Winnipeg, 515 Portage Avenue, Winnipeg, Manitoba, Canada R3B 2E9 Matthew R. M. Izawa Department of Geography, University of Winnipeg, 515 Portage Avenue, Winnipeg, Manitoba, Canada R3B 2E9 Paul Mann Department of Geography, University of Winnipeg, 515 Portage Avenue, Winnipeg, Manitoba, Canada R3B 2E9 Andreas Nathues Max-Planck-Institute for Solar System Research, Göttingen, Germany. 1 Pages: 62 Figures: 12 Tables: 4 Keywords: Asteroid Vesta; Asteroids, Composition; Asteroids, Surfaces; Mineralogy; Spectroscopy Proposed Running Head: Exogenic sources for the olivine on Vesta Editorial correspondence to: Lucille Le Corre Planetary Science Institute 1700 E Fort Lowell Rd #106 Tucson, Arizona 85719, USA [email protected] 2 Abstract The detection of olivine on Vesta is interesting because it may provide critical insights into planetary differentiation early in our Solar System’s history. Ground-based and Hubble Space Telescope (HST) observations of asteroid (4) Vesta have suggested the presence of olivine on the surface. These observations were reinforced by the discovery of olivine-rich HED meteorites from Vesta in recent years. However, analysis of data from NASA’s Dawn spacecraft has shown that this “olivine-bearing unit” is actually impact melt in the ejecta of Oppia crater. -

Sulfur on Mars from the Atmosphere to the Core Heather B
Sulfur on Mars from the Atmosphere to the Core Heather B. Franz1, Penelope L. King2, and Fabrice Gaillard3 1NASA Goddard Space Flight Center, Greenbelt, MD 20771, USA 2Research School of Earth Sciences, Australian National University, Canberra ACT 2601, Australia 3CNRS-Université d’Orléans, ISTO, la rue de la Ferollerie, 45071 Orléans, France Abstract Observations of the martian surface from orbiting spacecraft and in situ landers and rovers, as well as analyses of martian meteorites in terrestrial laboratories, have consistently indicated that Mars is a sulfur-rich planet. The global inventory of sulfur, from the atmosphere to the core, carries widespread implications of potential geophysical, geochemical, climatological, and astrobiological significance. For example, the sulfur content of the core carries implications for core density; the speciation of igneous sulfur minerals reflects the oxidation state of the magma from which they formed; sulfur-bearing gases may have exerted control on the temperatures at the surface of early Mars; and the widespread availability of sulfur on Mars would have provided an abundant source for energy and nutrients to fuel sulfur-metabolizing microbes, such as those that arose during the emergence of primitive life on Earth. Here we provide an overview of martian sulfur and its relevance to these areas of interest, including a discussion of analytical techniques and results acquired by space missions and meteorite analyses to date. We review current studies modeling the potential effects of sulfur-bearing gases on the past martian climate and possible constraints on atmospheric composition implied by sulfur isotopic data. We also explore the importance of sulfur to the search for extinct or extant life on Mars.