Geochemistry of the Martian Meteorite ALH84001, Revisited Jean-Alix Barrat, Claire Bollinger
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Origin of Ureilites Inferred from a SIMS Oxygen Isotopic and Trace Element Study of Clasts in the Dar Al Gani 319 Polymict Ureilite
Geochimica et Cosmochimica Acta, Vol. 68, No. 20, pp. 4213-4235, 2004 Copyright © 2004 Elsevier Ltd Pergamon Printed in the USA. All rights reserved 0016-7037/04 $30.00 ϩ .00 doi:10.1016/j.gca.2004.03.020 Origin of ureilites inferred from a SIMS oxygen isotopic and trace element study of clasts in the Dar al Gani 319 polymict ureilite 1,†, 2 1 1,‡ 1 3,4 NORIKO T. KITA, *YUKIO IKEDA, SHIGEKO TOGASHI, YONGZHONG LIU, YUICHI MORISHITA, and MICHAEL K. WEISBERG 1Geological Survey of Japan, AIST, AIST Central 7, Tsukuba 305-8567, Japan 2Faculty of Science, Ibaraki University, Mito 301-8512, Japan 3Department of Physical Sciences, Kingsborough College (CUNY), 2001 Oriental Boulevard, Brooklyn, NY 11235, USA 4Department of Earth and Planetary Sciences, American Museum of Natural History, New York, NY 10024, USA (Received June 12, 2003; accepted in revised form March 3, 2004) Abstract—Secondary ion mass spectrometer (SIMS) oxygen isotope analyses were performed on 24 clasts, representing 9 clast types, in the Dar al Gani (DaG) 319 polymict ureilite with precisions better than 1‰. Olivine-rich clasts with typical ureilitic textures and mineral compositions have oxygen isotopic compositions that are identical to those of the monomict ureilites and plot along the CCAM (Carbonaceous Chondrite Anhydrous Mineral) line. Other igneous clasts, including plagioclase-bearing clasts, also plot along the CCAM line, indicating that they were derived from the ureilite parent body (UPB). Thus, we suggest that some of the plagioclase-bearing clasts in the polymict ureilites represent the “missing basaltic component” produced by partial melting on the UPB. -

Shergotty Basalt, 5 Kg Seen to Fall Introduction the Shergotty Achondrite Fell on August 25, 1865 at 9:00 A.M
V. Shergotty basalt, 5 kg seen to fall Introduction The Shergotty achondrite fell on August 25, 1865 at 9:00 a.m. near a town called Shergahti in Bihar State, India after detonations were heard (Graham et al. 1985). Duke (1968) refers to several stones with fusion crusts, but this has not been confirmed. The main mass is at the Museum of the Geological Survey in Calcutta, India (figure V-1). In 1984, an international consortium was organized by J. C. Laul to study ~30 grams of Shergotty in detail (Laul 1986a, b). Shergottites (Shergotty, Zagami, EETA79001B, QUE94201, Los Angeles) are texturally and mineralogically similar to terrestrial diabases (although all of the plagioclase has been shocked to maskelynite), but quite distinct petrologically and chemically from the rest of the basaltic achondrites (Stolper et al. 1979). Stolper and McSween (1979) and others have noted that Shergotty crystallized under relatively oxidizing conditions. Figure V-1. Photograph of Shergotty meteorite The Shergotty meteorite has been severely shocked and showing fusion crust and borken surfaces. Two saw is considered the “type locality” for maskelynite (dense cuts are visible. Sample is about 25 cm across. Photo plagioclase glass). In fact, it has proven to be very kindly provided by Prof. N. Bhandari, Director, Physical Research Laboratory, Ahmedabad, India. difficult to date the original crystallization event of Figure V-2. Photograph of slab of Shergotty meteorite showing basaltic texture and alignment of pyroxene crystals. Note the inclusion of black glass in the center of this slab. This is figure 1 in Duke (1968). Photo courtesy of U. -
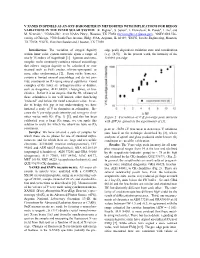
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Cosmic-Ray-Exposure Ages Martian Meteorites Million Years
Introduction to MMC 2012 someone keeps adding pieces as you go along (see my Introduction 2006). Most Martian meteorites are mafic As of the end of 2012, we now know of about 65 basalts. That is, they have an abundance of Fe and different meteorites from Mars with a total weight about Mg, are low in Si and Al and have a texture of 120 kilograms. Some fell as showers (e.g. Nakhla, intergrown minerals typical of terrestrial basalts (the Tissint), and some can be paired by common lithology, tradition has been to call them “shergottites”, after the age and fall location, but it is more instructive to first sample recognized as such). Some often have an consider how they are grouped by “launch pairs”. If abundance of large olivine crystals (so called olivine- you add the terrestrial age to the cosmic exposure age phyric). Others have an abundance of both high- and (CRE) you get the time when the meteorite was low-Ca pyroxene, with poikilitic textures and were launched off of Mars by impact. Since only rather large originally called “lherzolitic shergottites”. But it has impacts would do this, there must be a finite number now been recognized that there is a more fundamental of such impacts – hence grouping of samples. and better way to describe these rocks (Irving 2012). Now that we have such a large number of samples, it Figure 1 shows a crude summary of CRE that have is time to understand what we have and infer what we been determined for Martian meteorites. Note that can about Mars. -

Lafayette - 800 Grams Nakhlite
Lafayette - 800 grams Nakhlite Figure 1. Photograph showing fine ablation features Figure 2. Photograph of bottom surface of Lafayette of fusion crust on Lafayette meteorite. Sample is meteorite. Photograph from Field Museum Natural shaped like a truncated cone. This is a view of the top History, Chicago, number 62918. of the cone. Sample is 4-5 centimeters across. Photo- graph from Field Museum Natural History, Chicago, number 62913. Introduction According to Graham et al. (1985), “a mass of about 800 grams was noticed by Farrington in 1931 in the geological collections in Purdue University in Lafayette Indiana.” It was first described by Nininger (1935) and Mason (1962). Lafayette is very similar to the Nakhla and Governador Valadares meteorites, but apparently distinct from them (Berkley et al. 1980). Lafayette is a single stone with a fusion crust showing Figure 3. Side view of Lafayette. Photograph from well-developed flow features from ablation in the Field Museum Natural History, Chicago, number Earth’s atmosphere (figures 1,2,3). The specimen is 62917. shaped like a rounded cone with a blunt bottom end. It was apparently oriented during entry into the Earth’s that the water released during stepwise heating of atmosphere. Note that the fine ablation features seen Lafayette was enriched in deuterium. The alteration on Lafayette have not been reported on any of the assemblages in Lafayette continue to be an active field Nakhla specimens. of research, because it has been shown that the alteration in Lafayette occurred on Mars. Karlsson et al. (1992) found that Lafayette contained the most extra-terrestrial water of any Martian Lafayette is 1.32 b.y. -

Assessment of the Mesosiderite-Diogenite Connection and an Impact Model for the Genesis of Mesosiderites
45th Lunar and Planetary Science Conference (2014) 2554.pdf ASSESSMENT OF THE MESOSIDERITE-DIOGENITE CONNECTION AND AN IMPACT MODEL FOR THE GENESIS OF MESOSIDERITES. T. E. Bunch1,3, A. J. Irving2,3, P. H. Schultz4, J. H. Wittke1, S. M. Ku- ehner2, J. I. Goldstein5 and P. P. Sipiera3,6 1Dept. of Geology, SESES, Northern Arizona University, Flagstaff, AZ 86011 ([email protected]), 2Dept. of Earth & Space Sciences, University of Washington, Seattle, WA, 3Planetary Studies Foundation, Galena, IL, 4Dept. of Geological Sciences, Brown University, Providence, RI, 5Dept. of Geolo- gy, University of Massachusetts, Amherts, MA, 6Field Museum of Natural History, Chicago, IL. Introduction: Among well-recognized meteorite 34) is the most abundant silicate mineral and in some classes, the mesosiderites are perhaps the most com- clasts contains inclusions of FeS, tetrataenite, merrillite plex and petrogenetically least understood. Previous and silica. Three of the ten norite clasts contain a few workers have contributed important information about tiny grains of olivine (Fa24-32). A single, fine-grained “classic” falls and Antarctic finds, and have proposed breccia clast was found in NWA 5312 (see Figure 2). several different models for mesosiderite genesis [1]. Unlike the case of pallasites, the co-occurrence of met- al and silicates (predominantly orthopyroxene and cal- cic plagioclase) in mesosiderites is inconsistent with a single-stage “igneous” history, and instead seems to demand admixture of at least two separate compo- nents. Here we review the models in light of detailed ex- amination of multiple specimens from a very large mesosiderite strewnfield in Northwest Africa. Many specimens (totaling at least 80 kilograms) from this area (probably in Algeria) have been classified sepa- rately by us and others; however, in most cases the Figure 1. -

Sequestration of Martian CO2 by Mineral Carbonation
ARTICLE Received 11 Jun 2013 | Accepted 24 Sep 2013 | Published 22 Oct 2013 DOI: 10.1038/ncomms3662 OPEN Sequestration of Martian CO2 by mineral carbonation Tim Tomkinson1, Martin R. Lee2, Darren F. Mark1 & Caroline L. Smith3,4,5 Carbonation is the water-mediated replacement of silicate minerals, such as olivine, by carbonate, and is commonplace in the Earth’s crust. This reaction can remove significant quantities of CO2 from the atmosphere and store it over geological timescales. Here we present the first direct evidence for CO2 sequestration and storage on Mars by mineral carbonation. Electron beam imaging and analysis show that olivine and a plagioclase feldspar- rich mesostasis in the Lafayette meteorite have been replaced by carbonate. The susceptibility of olivine to replacement was enhanced by the presence of smectite veins along which CO2-rich fluids gained access to grain interiors. Lafayette was partially carbonated during the Amazonian, when liquid water was available intermittently and atmospheric CO2 concentrations were close to their present-day values. Earlier in Mars’ history, when the planet had a much thicker atmosphere and an active hydrosphere, carbonation is likely to have been an effective mechanism for sequestration of CO2. 1 Scottish Universities Environmental Research Centre, Rankine Avenue, Scottish Enterprise Technology Park, East Kilbride G75 0QF, UK. 2 School of Geographical and Earth Sciences, University of Glasgow, Gregory Building, Lilybank Gardens, Glasgow G12 8QQ, UK. 3 Department of Earth Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK. 4 ESA ESTEC, Keplerlaan 1, 200 AG Noordwijk, The Netherlands. 5 UK Space Agency, Atlas Building, Harwell Oxford, Didcot, Oxfordshire OX11 0QX, UK. -

Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975
Originally published as: Słaby, E., Förster, H.‐J., Wirth, R., Giera, A., Birski, Ł., Moszumańska, I. (2017): Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975. ‐ Geosciences, 7, 4. DOI: http://doi.org/10.3390/geosciences7040099 geosciences Article Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975 Ewa Słaby 1,*, Hans-Jürgen Förster 2, Richard Wirth 2, Alicja Wudarska 1,2, Łukasz Birski 1 and Izabela Moszuma ´nska 1 1 Institute of Geological Sciences, Polish Academy of Sciences, Research Centre in Warsaw, Twarda 51/55, 00-818 Warsaw, Poland; [email protected] (A.W.); [email protected] (Ł.B.); [email protected] (I.M.) 2 Helmholtz-Zentrum Potsdam Deutsches GeoForschungsZentrum GFZ, Telegrafenberg, 14473 Potsdam, Germany; [email protected] (H.-J.F.); [email protected] (R.W.) * Correspondence: [email protected] Received: 17 July 2017; Accepted: 26 September 2017; Published: 4 October 2017 Abstract: Phosphates from the Martian shergottite NWA 2975 were used to obtain insights into the source and subsequence differentiation of the melt/melts. The crystallization of two generations of fluorapatite (F > Cl~OH and F-rich), chlorapatite and ferromerrillite-merrillite were reconstructed from TEM (Transmission Electron Microscopy) and geochemical analyses. The research results indicated that the recognized volatiles budget of the two generations of fluorapatite was related to their magmatic origin. The apatite crystals crystallized from an evolved magma during its final differentiation and degassing stage. -

Secondary Minerals in the Nakhlite Meteorite Yamato 000593: Distinguishing Martian from Terrestrial Alteration Products
46th Lunar and Planetary Science Conference (2015) 2010.pdf SECONDARY MINERALS IN THE NAKHLITE METEORITE YAMATO 000593: DISTINGUISHING MARTIAN FROM TERRESTRIAL ALTERATION PRODUCTS. H. Breton1, M. R. Lee1, and D. F. Mark2 1School of Geographical and Earth Sciences, University of Glasgow, University Ave, Glasgow, Lanarkshire G12 8QQ, UK ([email protected]), 2Scottish Universities Environmental Research Center, Rankine Ave, Scottish Enterprise Technology Park, East Kilbride G75 0QF, UK Introduction: The nakhlites are olivine-bearing Methods: A thin section of Y-000593 was studied clinopyroxenites that formed in a Martian lava flow or using a Carl Zeiss Sigma field-emission SEM equipped shallow intrusion 1.3 Ga ago [1, 2]. They are scientifi- with an Oxford Instruments Aztec microanalysis sys- cally extremely valuable because they interacted with tem at the University of Glasgow. Chemical and miner- water-bearing fluids on Mars [3]. Fluid-rock interac- alogical identification within the secondary minerals tions led to the precipitation of secondary minerals, were obtained through backscattered electron (BSE) many of which are hydrous. The secondary minerals imaging and energy dispersive spectroscopy (EDS) consist in a mixture of poorly crystalline smectitic ma- mapping and quantitative microanalysis. terial and Fe-oxide, collectively called “iddingsite”, but Results and discussions: Y-000593 is an unbrec- also carbonate and sulphate [4]. The proportion, chem- ciated cumulate rock whose mineralogy is similar to istry and habit of the secondary minerals vary between other nakhlites: a predominance of augite and minor members of the Nakhlite group, which is thought to olivine phenocrysts surrounded by a microcrystalline reflect compositional variation of the fluid within the mesostasis [9]. -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

MARTIAN METEORITES: GEOCHEMISTRY and SUCH 6:00 P.M
Lunar and Planetary Science XLVIII (2017) sess333.pdf Tuesday, March 21, 2017 [T333] POSTER SESSION I: MARTIAN METEORITES: GEOCHEMISTRY AND SUCH 6:00 p.m. Town Center Exhibit Area Irving A. J. Kuehner S. M. Lapen T. J. Righter M. Busemann H. et al. POSTER LOCATION #466 Keeping Up with the Martian Meteorites and Constraining the Number of Separate Launch Sites on Mars [#2068] Petrologic, chemical, and cosmogenic nuclide criteria suggest that the 101 unpaired martian meteorites may come from as few as 20 launch sites on Mars. Habermann M. Agee C. Spilde M. POSTER LOCATION #467 Multi-Layered Clast in Martian Breccia Northwest Africa 10922 [#2743] We performed chemical analyses of an unusual, concentrically-layered clast within the martian breccia NWA 10922. Santos A. R. Lewis J. A. Agee C. B. Humayun M. McCubbin F. M. et al. POSTER LOCATION #468 Feldspar Variability in Northwest Africa 7034 [#2349] Northwest Africa 7034 contains feldspar of different grain size, texture, and composition, some of which suggest modification by secondary alteration. Cao T. He Qi. POSTER LOCATION #469 Petrology and Mineral Chemistry of the Eniched Basaltic Shergottite Northwest Africa 8656 [#1384] The characterization of primary petrography and mineralogy of Northwest Africa 8656, basalt shergottite, are described in this abstract. Jacobs G. M. Abernethey F. J. Anand M. Franchi I. A. Grady M. M. POSTER LOCATION #470 Understanding the Early Martian Environment Through the Inventory of Breccia Clasts in Northwest Africa 7034 [#2139] The clasts and matrices of Northwest Africa 7034 and its breccia clasts reveal the meteorite aggregates material from multiple sources with distinct histories. -

(M = Ca, Mg, Fe2+), a Structural Base of Ca3mg3(PO4)4 Phosphors
crystals Article Crystal Chemistry of Stanfieldite, Ca7M2Mg9(PO4)12 (M = Ca, Mg, Fe2+), a Structural Base of Ca3Mg3(PO4)4 Phosphors Sergey N. Britvin 1,2,* , Maria G. Krzhizhanovskaya 1, Vladimir N. Bocharov 3 and Edita V. Obolonskaya 4 1 Department of Crystallography, Institute of Earth Sciences, St. Petersburg State University, Universitetskaya Nab. 7/9, 199034 St. Petersburg, Russia; [email protected] 2 Nanomaterials Research Center, Kola Science Center of Russian Academy of Sciences, Fersman Str. 14, 184209 Apatity, Russia 3 Centre for Geo-Environmental Research and Modelling, Saint-Petersburg State University, Ulyanovskaya ul. 1, 198504 St. Petersburg, Russia; [email protected] 4 The Mining Museum, Saint Petersburg Mining University, 2, 21st Line, 199106 St. Petersburg, Russia; [email protected] * Correspondence: [email protected] Received: 1 May 2020; Accepted: 25 May 2020; Published: 1 June 2020 Abstract: Stanfieldite, natural Ca-Mg-phosphate, is a typical constituent of phosphate-phosphide assemblages in pallasite and mesosiderite meteorites. The synthetic analogue of stanfieldite is used as a crystal matrix of luminophores and frequently encountered in phosphate bioceramics. However, the crystal structure of natural stanfieldite has never been reported in detail, and the data available so far relate to its synthetic counterpart. We herein provide the results of a study of stanfieldite from the Brahin meteorite (main group pallasite). The empirical formula of the mineral is Ca8.04Mg9.25Fe0.72Mn0.07P11.97O48. Its crystal structure has been solved and refined to R1 = 0.034. Stanfieldite from Brahin is monoclinic, C2/c, a 22.7973(4), b 9.9833(2), c 17.0522(3) Å, β 99.954(2)◦, 3 V 3822.5(1)Å .