02 Rutherford Scattering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -

Compton Scattering from the Deuteron at Low Energies
SE0200264 LUNFD6-NFFR-1018 Compton Scattering from the Deuteron at Low Energies Magnus Lundin Department of Physics Lund University 2002 W' •sii" Compton spridning från deuteronen vid låga energier (populärvetenskaplig sammanfattning på svenska) Vid Compton spridning sprids fotonen elastiskt, dvs. utan att förlora energi, mot en annan partikel eller kärna. Kärnorna som användes i detta försök består av en proton och en neutron (sk. deuterium, eller tungt väte). Kärnorna bestrålades med fotoner av kända energier och de spridda fo- tonerna detekterades mha. stora Nal-detektorer som var placerade i olika vinklar runt strålmålet. Försöket utfördes under 8 veckor och genom att räkna antalet fotoner som kärnorna bestålades med och antalet spridda fo- toner i de olika detektorerna, kan sannolikheten för att en foton skall spridas bestämmas. Denna sannolikhet jämfördes med en teoretisk modell som beskriver sannolikheten för att en foton skall spridas elastiskt mot en deuterium- kärna. Eftersom protonen och neutronen består av kvarkar, vilka har en elektrisk laddning, kommer dessa att sträckas ut då de utsätts för ett elek- triskt fält (fotonen), dvs. de polariseras. Värdet (sannolikheten) som den teoretiska modellen ger, beror på polariserbarheten hos protonen och neu- tronen i deuterium. Genom att beräkna sannolikheten för fotonspridning för olika värden av polariserbarheterna, kan man se vilket värde som ger bäst överensstämmelse mellan modellen och experimentella data. Det är speciellt neutronens polariserbarhet som är av intresse, och denna kunde bestämmas i detta arbete. Organization Document name LUND UNIVERSITY DOCTORAL DISSERTATION Department of Physics Date of issue 2002.04.29 Division of Nuclear Physics Box 118 Sponsoring organization SE-22100 Lund Sweden Author (s) Magnus Lundin Title and subtitle Compton Scattering from the Deuteron at Low Energies Abstract A series of three Compton scattering experiments on deuterium have been performed at the high-resolution tagged-photon facility MAX-lab located in Lund, Sweden. -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

12 Scattering in Three Dimensions
12 Scattering in three dimensions 12.1 Cross sections and geometry Most experiments in physics consist of sending one particle to collide with another, and looking at what comes out. The quantity we can usually measure is the scattering cross section: by analogy with classical scattering of hard spheres, we assuming that scattering occurs if the particles ‘hit’ each other. The cross section is the apparent ‘target area’. The total scattering cross section can be determined by the reduction in intensity of a beam of particles passing through a region on ‘targets’, while the differential scattering cross section requires detecting the scattered particles at different angles. We will use spherical polar coordinates, with the scattering potential located at the origin and the plane wave incident flux parallel to the z direction. In this coordinate system, scattering processes dσ are symmetric about φ, so dΩ will be independent of φ. We will also use a purely classical concept, the impact parameter b which is defined as the distance of the incident particle from the z-axis prior to scattering. S(k) δΩ I(k) θ z φ Figure 11: Standard spherical coordinate geometry for scattering 12.2 The Born Approximation We can use time-dependent perturbation theory to do an approximate calculation of the cross- section. Provided that the interaction between particle and scattering centre is localised to the region around r = 0, we can regard the incident and scattered particles as free when they are far from the scattering centre. We just need the result that we obtained for a constant perturbation, Fermi’s Golden Rule, to compute the rate of transitions between the initial state (free particle of momentum p) to the final state (free particle of momentum p0). -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Calculation of Photon Attenuation Coefficients of Elements And
732 ISSN 0214-087X Calculation of photon attenuation coeffrcients of elements and compound Roteta, M.1 Baró, 2 Fernández-Varea, J.M.3 Salvat, F.3 1 CIEMAT. Avenida Complutense 22. 28040 Madrid, Spain. 2 Servéis Científico-Técnics, Universitat de Barcelona. Martí i Franqués s/n. 08028 Barcelona, Spain. 3 Facultat de Física (ECM), Universitat de Barcelona. Diagonal 647. 08028 Barcelona, Spain. CENTRO DE INVESTIGACIONES ENERGÉTICAS, MEDIOAMBIENTALES Y TECNOLÓGICAS MADRID, 1994 CLASIFICACIÓN DOE Y DESCRIPTORES: 990200 662300 COMPUTER LODES COMPUTER CALCULATIONS FORTRAN PROGRAMMING LANGUAGES CROSS SECTIONS PHOTONS Toda correspondencia en relación con este trabajo debe dirigirse al Servicio de Información y Documentación, Centro de Investigaciones Energéticas, Medioam- bientales y Tecnológicas, Ciudad Universitaria, 28040-MADRID, ESPAÑA. Las solicitudes de ejemplares deben dirigirse a este mismo Servicio. Los descriptores se han seleccionado del Thesauro del DOE para describir las materias que contiene este informe con vistas a su recuperación. La catalogación se ha hecho utilizando el documento DOE/TIC-4602 (Rev. 1) Descriptive Cataloguing On- Line, y la clasificación de acuerdo con el documento DOE/TIC.4584-R7 Subject Cate- gories and Scope publicados por el Office of Scientific and Technical Information del Departamento de Energía de los Estados Unidos. Se autoriza la reproducción de los resúmenes analíticos que aparecen en esta publicación. Este trabajo se ha recibido para su impresión en Abril de 1993 Depósito Legal n° M-14874-1994 ISBN 84-7834-235-4 ISSN 0214-087-X ÑIPO 238-94-013-4 IMPRIME CIEMAT Calculation of photon attenuation coefíicients of elements and compounds from approximate semi-analytical formulae M. -

Rutherford's Atomic Model
CHAPTER 4 Structure of the Atom 4.1 The Atomic Models of Thomson and Rutherford 4.2 Rutherford Scattering 4.3 The Classic Atomic Model 4.4 The Bohr Model of the Hydrogen Atom 4.5 Successes and Failures of the Bohr Model 4.6 Characteristic X-Ray Spectra and Atomic Number 4.7 Atomic Excitation by Electrons 4.1 The Atomic Models of Thomson and Rutherford Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit: 1) There seemed to be too many kinds of atoms, each belonging to a distinct chemical element. 2) Atoms and electromagnetic phenomena were intimately related. 3) The problem of valence (원자가). Certain elements combine with some elements but not with others, a characteristic that hinted at an internal atomic structure. 4) The discoveries of radioactivity, of x rays, and of the electron Thomson’s Atomic Model Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom with, the newly discovered “negative” electrons embedded in the uniform background. In Thomson’s view, when the atom was heated, the electrons could vibrate about their equilibrium positions, thus producing electromagnetic radiation. Experiments of Geiger and Marsden Under the supervision of Rutherford, Geiger and Marsden conceived a new technique for investigating the structure of matter by scattering particles (He nuclei, q = +2e) from atoms. Plum-pudding model would predict only small deflections. (Ex. 4-1) Geiger showed that many particles were scattered from thin gold-leaf targets at backward angles greater than 90°. -
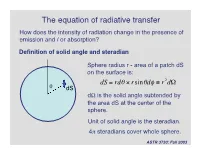
The Equation of Radiative Transfer How Does the Intensity of Radiation Change in the Presence of Emission and / Or Absorption?
The equation of radiative transfer How does the intensity of radiation change in the presence of emission and / or absorption? Definition of solid angle and steradian Sphere radius r - area of a patch dS on the surface is: dS = rdq ¥ rsinqdf ≡ r2dW q dS dW is the solid angle subtended by the area dS at the center of the † sphere. Unit of solid angle is the steradian. 4p steradians cover whole sphere. ASTR 3730: Fall 2003 Definition of the specific intensity Construct an area dA normal to a light ray, and consider all the rays that pass through dA whose directions lie within a small solid angle dW. Solid angle dW dA The amount of energy passing through dA and into dW in time dt in frequency range dn is: dE = In dAdtdndW Specific intensity of the radiation. † ASTR 3730: Fall 2003 Compare with definition of the flux: specific intensity is very similar except it depends upon direction and frequency as well as location. Units of specific intensity are: erg s-1 cm-2 Hz-1 steradian-1 Same as Fn Another, more intuitive name for the specific intensity is brightness. ASTR 3730: Fall 2003 Simple relation between the flux and the specific intensity: Consider a small area dA, with light rays passing through it at all angles to the normal to the surface n: n o In If q = 90 , then light rays in that direction contribute zero net flux through area dA. q For rays at angle q, foreshortening reduces the effective area by a factor of cos(q). -

Application of Ion Scattering Techniques to Characterize Polymer Surfaces and Interfaces
Materials Science and Engineering R 38 (2002) 107±180 Application of ion scattering techniques to characterize polymer surfaces and interfaces Russell J. Compostoa,*, Russel M. Waltersb, Jan Genzerc aLaboratory for Research on the Structure of Matter, Department of Materials Science and Engineering, University of Pennsylvania, Philadelphia, PA 19104-6272, USA bDepartment of Chemical Engineering, University of Pennsylvania, Philadelphia, PA 19104-6272, USA cDepartment of Chemical Engineering, North Carolina State University, Raleigh, NC 27695-7905, USA Abstract Ion beam analysis techniques, particularly elastic recoil detection (ERD) also known as forward recoil spectrometry (Frcs) has proven to be a value tool to investigate polymer surfaces and interfaces. A review of ERD, related techniques and their impact on the field of polymer science is presented. The physics of the technique is described as well as the underlying principles of the interaction of ions with matter. Methods for optimization of ERD for polymer systems are also introduced, specifically techniques to improve the depth resolution and sensitivity. Details of the experimental setup and requirements are also laid out. After a discussion of ERD, strategies for the subsequent data analysis are described. The review ends with the breakthroughs in polymer science that ERD enabled in polymer diffusion, surfaces, interfaces, critical phenomena, and polymer modification. # 2002 Elsevier Science B.V. All rights reserved. Keywords: Ion scattering; Polymer surface; Polymer interface; Elastic recoil detection; Forward recoil spectrometry; Depth profiling 1. Introduction Breakthroughs in polymer science typically correlate with the discovery and application of new experimental tools. One of the first examples was the prediction by P.J. Flory that the single chain conformation in a dense system (i.e. -

Rutherford Scattering
Chapter 5 Lecture 4 Rutherford Scattering Akhlaq Hussain 1 5.5 Rutherford Scattering Formula Rutherford’s experiment of 훼-particles scattering by the atoms in a thin foil of gold revealed the existence of positively charged nucleus in the atom. ➢ The 훼-particles were scattered in all directions. ➢ Some passes undeflected by the foil. ➢ Some were scattered through larger angles even back scattered. ➢ The only valid explanation of large angle deflection was if the total positive charge were concentrated at some small region. ➢ Rutherford called it nucleus. 2 5.5 Rutherford Scattering Formula ′ 풖ퟏ 3 5.5 Rutherford Scattering Formula Consider an incident charge particle with charge z1e is scattered through a target with charge z2e. The trajectory of scattered particles is a hyperbola as shown. The trajectory of the particles in repulsive field with origin at o' is shown. In the case of attractive force, center will be at “o”. when particles are apart at large distance, the total energy it posses is in form of Kinetic energy ′ 1 ′ 2 푘 = ൗ2 µ푢1 = 퐸 ′ 2퐸 푢1 = µ 4 5.5 Rutherford Scattering Formula 푆 푑푠 We have to find 휎 휃′ = 푠푖푛 휃′ 푑휃′ ′ 푙 = µ푢1푆 = 푆 2µ퐸 Where S is the impact parameter. The trajectory is hyperbola, from equation of conic 훼 = 푒 cos 훼 − 1 and for 푟 → ∞ 푟 1 1 ⇒ cos 훼 = = 푒 2퐸푙2 1+ µ푘2 From the figure 2훼 + 휃′ = 휋 휋−휃′ 훼 = 2 휋−휃′ 휃′ Now cot 훼 = cot = tan 5 2 2 5.5 Rutherford Scattering Formula 휃′ cot 훼 = tan 2 휃′ cos 훼 cos 훼 tan = = 2 sin 훼 1−푐표푠2훼 휃′ cos 훼 tan = 2 1 cos 훼 −1 푐표푠2훼 휃′ 1 tan = 2 1 −1 푐표푠2훼 휃′ 1 1 tan = = 2 푒2−1 2퐸푙2 1+ −1 휇푘2 6 5.5 Rutherford Scattering Formula −1Τ 휃′ 2퐸푙2 2 tan = 2 휇푘2 휃′ 푘 휇 푘 tan = = ′ 2퐸 2 푙 2퐸 푙푢′ Because 푢 = 1 1 µ ′ Since 푙 = µ푢1S 휃′ 푘 ⇒ tan = ′2 2 푆µ푢1 Differentiating with θ' above equation can be written as. -

Slides for Statistics, Precision, and Solid Angle
Slides for Statistics, Precision, and Solid Angle 22.01 – Intro to Radiation October 14, 2015 22.01 – Intro to Ionizing Radiation Precision etc., Slide 1 Solid Angles, Dose vs. Distance • Dose decreases with the inverse square of distance from the source: 1 퐷표푠푒 ∝ 푟2 • This is due to the decrease in solid angle subtended by the detector, shielding, person, etc. absorbing the radiation 22.01 – Intro to Ionizing Radiation Precision etc., Slide 2 Solid Angles, Dose vs. Distance • The solid angle is defined in steradians, and given the symbol Ω. • For a rectangle with width w and length l, at a distance r from a point source: 푤푙 Ω = 4푎푟푐푡푎푛 2푟 4푟2 + w2 + 푙2 • A full sphere has 4π steradians (Sr) 22.01 – Intro to Ionizing Radiation Precision etc., Slide 3 Solid Angles, Dose vs. Distance http://www.powerfromthesun.net/Book/chapter02/chapter02.html • Total luminance (activity) of a source is constant, but the flux through a surface decreases with distance Courtesy of William B. Stine. Used with permission. 22.01 – Intro to Ionizing Radiation Precision etc., Slide 4 Exponential Gamma Attenuation • Gamma sources are attenuated exponentially according to this formula: Initial intensity Mass attenuation coefficient 흁 Distance through − 흆 흆풙 푰 = 푰ퟎ풆 material Transmitted intensity Material density • Attenuation means removal from a narrowly collimated beam by any means 22.01 – Intro to Ionizing Radiation Precision etc., Slide 5 Exponential Gamma Attenuation Look up values in NIST x-ray attenuation tables: http://www.nist.gov/pml/data/xraycoef/ Initial -

3 Scattering Theory
3 Scattering theory In order to find the cross sections for reactions in terms of the interactions between the reacting nuclei, we have to solve the Schr¨odinger equation for the wave function of quantum mechanics. Scattering theory tells us how to find these wave functions for the positive (scattering) energies that are needed. We start with the simplest case of finite spherical real potentials between two interacting nuclei in section 3.1, and use a partial wave anal- ysis to derive expressions for the elastic scattering cross sections. We then progressively generalise the analysis to allow for long-ranged Coulomb po- tentials, and also complex-valued optical potentials. Section 3.2 presents the quantum mechanical methods to handle multiple kinds of reaction outcomes, each outcome being described by its own set of partial-wave channels, and section 3.3 then describes how multi-channel methods may be reformulated using integral expressions instead of sets of coupled differential equations. We end the chapter by showing in section 3.4 how the Pauli Principle re- quires us to describe sets identical particles, and by showing in section 3.5 how Maxwell’s equations for electromagnetic field may, in the one-photon approximation, be combined with the Schr¨odinger equation for the nucle- ons. Then we can describe photo-nuclear reactions such as photo-capture and disintegration in a uniform framework. 3.1 Elastic scattering from spherical potentials When the potential between two interacting nuclei does not depend on their relative orientiation, we say that this potential is spherical. In that case, the only reaction that can occur is elastic scattering, which we now proceed to calculate using the method of expansion in partial waves.