The IEA Weyburn CO2 Monitoring and Storage Project Final Report of the European Research Team
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Happens When CO2 Is Stored Underground? Q&A from the IEAGHG Weyburn-Midale CO2 Monitoring and Storage Project
WHAT HAPPENS WHEN CO2 IS STORED UNDERGROUND Q&A from the IEAGHG Weyburn-Midale CO2 Monitoring and Storage Project This report was developed by: What Happens When CO2 is Stored Underground? Q&A from the IEAGHG Weyburn-Midale CO2 Monitoring and Storage Project This document is also available in electronic format from the Global CCS Institute atwww.globalccsinsitute.com and from the Petroleum Technology Research Centre (PTRC) at http://ptrc.ca This paper was made possible through funding from the Global CCS Institute, in the interest of broadening the discussion on CCS as a key climate change mitigation option. The views contained in this paper do not necessarily represent those of the Global CCS Institute or its members. The Global CCS Institute does not give any representation or warranty as to the reliability, accuracy or completeness of the information, nor does it accept any responsibility arising in any way (including by negligence) for errors in, or omissions from, the information. © Global Carbon Capture and Storage Institute Limited 2014, Melbourne. Queries may be directed to: Global CCS Institute PO Box 23335 Docklands VIC 8012 Australia [email protected] and/or Petroleum Technology Research Centre 220, 6 Research Drive Regina, Saskatchewan, Canada S4S 7J7 [email protected] ISBN Number 978-0-9871873-3-1 1. Carbon capture and storage 2. Carbon dioxide sequestration 3. Carbon dioxide measurement and monitoring Front Cover: A CO2 injection well, covered in a fibreglass hut, rests in field near Weyburn, Saskatchewan. Back Cover: A worker inspects the Cenovus Energy Weyburn plant. (Photograph courtesy of Cenovus Energy) INTRODUCTION The IEA Greenhouse Gas Programme Weyburn-Midale Carbon Dioxide (CO2) Monitoring and Storage Project was a world-leading research program designed to examine the effects of injecting and storing carbon dioxide (CO2) into oil reservoirs in southeastern Saskatchewan, Canada. -

Hansard April 10, 2000
LEGISLATIVE ASSEMBLY OF SASKATCHEWAN 601 April 10, 2000 The Assembly met at 1:30 p.m. Mr. Boyd: — Thank you, Mr. Speaker. I’m pleased on behalf of the residents of Weyburn as well to present a petition in Prayers support of their court house: ROUTINE PROCEEDINGS Wherefore your petitioners humbly pray that your Hon. PRESENTING PETITIONS Assembly may be pleased to cause the government to continue the Court of Queen’s Bench services in the Mr. Elhard: — Thank you, Mr. Speaker. I rise this afternoon to community of Weyburn. present a petition on behalf of constituents of Cypress Hills in opposition to the concept of forced amalgamation of rural Mr. Gantefoer: — Mr. Speaker, I rise on behalf of citizens municipalities. concerned about the high price of fuel. The prayer reads as follows: And these signatories are from the communities of Gull Lake, and Tompkins, as well as Webb and Abbey. Wherefore your petitioners humbly pray that your Hon. Assembly may be pleased to cause the federal and I so do present. provincial governments to immediately reduce fuel taxes by 10 cents a litre, cost shared by both levels of Mr. Heppner: — Thank you, Mr. Speaker. I too rise to present government. a petition, and this one deals with some of the things that are happening in our province dealing with court houses. And I Signatures on this petition, Mr. Speaker, are from Saskatoon read the prayer: and Melfort. Wherefore your petitioners humbly pray that your Hon. I so present. Assembly may be pleased to cause the government to continue Court of Queen Bench services in the community Mr. -

The World's Largest CO2 Storage Research Project with EOR
The World's Largest CO2 Storage Research Project with EOR IEA GHG Weyburn-Midale CO Monitoring & Storage Introduction: IEA GHG Weyburn-Midale About the IEA GHG Weyburn-Midale CO2 2 The Facts about the IEA GHG Weyburn- Project: Final Phase CO2 Monitoring & Storage Project Monitoring & Storage Project Midale CO2 Monitoring & Storage Project Building on the positive results received from the first phase, the objective of the Final The Project is Recognized and Endorsed By: • An 8-year, $80 million project on carbon storage funded by partners around Phase is to encourage the widespread use of technologies required to design, implement, IEA GHG Weyburn-Midale CO2 Monitoring & Storage monitor and verify a significant number of CO geological storage projects throughout the the world 2 • The International Energy Agency Greenhouse Gas R&D Programme (ensuring world. Through both a technical component, led by the PTRC, and a public policy and • Investigates potential for storage of man-made CO2 in the enhanced oil recovery Project: First Phase technical excellence) community outreach component, managed by the Leading Sponsors, including Natural (EOR) process, from technical and regulatory perspectives • The Carbon Sequestration Leadership Forum (ensuring sound policy and Resources Canada and the US Department of Energy-NETL, the second phase will • The world's largest, full-scale, in-field Measurement Monitoring and The first phase began in 2000 and ended in 2004. The purpose of the first phase was to regulation development) Verification study with EOR predict and verify the ability of an oil reservoir to securely and economically store and further carbon storage in Canada by: • First phase led by the Petroleum Technology Research Centre (PTRC), which contain CO2. -

Greenhouse Gas Sequestration In
was initiated to investigate the technical Greenhouse gas sequestration and economic feasibility of CO2 stor- age in a partially depleted oil reservoir (Government of Canada, 2000). The IEA in abandoned oil reservoirs: Weyburn project is exploiting EnCana Corporation’s $1.5 billion, 30-year com- The International Energy Agency mercial CO2 enhanced oil recovery operation, which is designed to recover an incremental 130 million barrels of oil Weyburn pilot project from the Weyburn field through the in- jection of gaseous CO2 under pressure. D.J. White, Geological Survey of Canada, 615 Booth Street, Ottawa, Ontario K1A 0E9, Specifically, the IEA Weyburn Project Canada, [email protected] aims to comprehensively monitor and verify the progress of the CO flood G. Burrowes, EnCana Corporation, 421 7th Avenue SW, P.O. Box 2850, 2 and establish the likelihood of safely Calgary, Alberta T2P 2S5, Canada storing the CO2 in the reservoir for the T. Davis, Colorado School of Mines, Golden, Colorado 80401-1887, USA long term. Toward this end, a multidis- Z. Hajnal, Department of Geological Sciences, University of Saskatchewan, ciplinary, integrated program has been Saskatoon, Saskatchewan S7N 0W0, Canada formulated to address critical issues cen- tral to safe and cost-effective, long-term K. Hirsche, Hampson-Russell Software, 715 5th Avenue SW, Calgary, storage of CO . In this article, we focus Alberta T2P 2X6, Canada 2 on the regional geoscience framework I. Hutcheon, Department of Geology and Geophysics, University of Calgary, and the monitoring and verification Calgary, Alberta, T2N 1N4, Canada components of the project. E. Majer, 90-MS1116, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA GEOLOGICAL SETTING AND HISTORY OF THE WEYBURN FIELD B. -

Business Development Manager Radius Credit Union Limited – Radville Branch
Career Opportunity Business Development Manager Radius Credit Union Limited – Radville Branch Job Profile: Radius Credit Union is seeking a full time Business Development Manager to be based out of our Radville Branch. Reporting to the Manager of Retail Operations, this position is responsible for managing the operations of a branch team in order to provide products and services to members to ensure the achievement of the organization’s goals and strategies. In addition to managing the day-to-day operations of the assigned branches, this position will focus on business development by building long-term financial relationships with members through the delivery of deposit products and lending services to meet the members’ needs. Responsible for a full range of performance management including goal setting, coaching, development and evaluating performance; motivation and recognition of staff; determining staffing, scheduling and training needs. Qualifications: Our successful candidate will have seven plus years of related experience and/or education in the financial industry. Ideal qualities include: • High-level knowledge of financial industry, trends and credit union advantages. • Proficient in business development and leadership. • Lending experience is an asset; working knowledge and understanding of the legal requirements associated with lending. • Wealth Management & Investment experience is an asset including working knowledge and understanding of investment services and registered products. • A commitment to self-development, self-motivated and able to work independently to guide a team of individuals to ensure branch activities align with the strategic corporate objectives. • A collaborative work style and excellent communication and interpersonal skills with a desire to share time, expertise and success with others. -

The Kerr Investigation: Executive Summary
THE KERR INVESTIGATION: EXECUTIVE SUMMARY The Kerr Investigation: Final Report | 2 The Kerr Investigation: Final Report | 3 THE KERR INVESTIGATION: EXECUTIVE SUMMARY CLIMATE CHANGE AND CO2 Carbon dioxide (CO2) is a greenhouse gas. While carbon dioxide emissions naturally occur in the environment, there is a widespread belief that human activities are disturbing the natural exchange of carbon between the geosphere, the biosphere, the oceans and the atmosphere. The burning of fossil fuels for power production, heating, industry and transportation continue to raise the level of CO2 in the atmosphere. CO2 accumulates in the atmosphere resulting in climate change by trapping a portion of the sun’s heat and causing the earth’s surface to warm. In 2007, the Intergovernmental Panel on Climate Change (IPCC), representing 2,500 scientists from more than 30 countries, concluded that there is 90 per cent likelihood that global warming has been caused by human industrial activity. The Panel identified Carbon Capture and Storage (CCS) as one of the most promising near term technologies for the rapid reduction of global CO2 emissions. This fall an in-depth study conducted by the Berkeley Earth Surface Temperature project verified these results. A scientific team led by physics professor Richard Muller of the University of California, Berkley and senior faculty scientists at the Lawrence Berkley National Laboratory confirmed that the Earth’s temperature has risen by 1 degree Celsius since the 1950s. The research team compiled information available from 1800 to 2009. Today, scientists, governments and industry worldwide are working together to reduce the impact of CO2 on Earth’s environment, stopping potentially irreversible climate changes. -

Using Noble Gas Fingerprints at the Kerr Farm to Assess CO2 Leakage Allegations Linked to the Weyburn-Midale CO2 Monitoring and Storage Project
Edinburgh Research Explorer Using noble gas fingerprints at the Kerr Farm to assess CO2 leakage allegations linked to the Weyburn-Midale CO2 Monitoring and Storage Project Citation for published version: Gilfillan, S, Sherk, GW, Poreda, RJ & Haszeldine, R 2017, 'Using noble gas fingerprints at the Kerr Farm to assess CO2 leakage allegations linked to the Weyburn-Midale CO2 Monitoring and Storage Project', International Journal of Greenhouse Gas Control, vol. 63, pp. 215-225. https://doi.org/10.1016/j.ijggc.2017.05.015 Digital Object Identifier (DOI): 10.1016/j.ijggc.2017.05.015 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: International Journal of Greenhouse Gas Control General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 30. Sep. 2021 International Journal of Greenhouse Gas Control 63 (2017) 215–225 Contents lists available at ScienceDirect International Journal of Greenhouse Gas Control journal homepage: www.elsevier.com/locate/ijggc Using noble gas fingerprints at the Kerr Farm to assess CO2 leakage MARK allegations linked to the Weyburn-Midale CO2 monitoring and storage project ⁎ Stuart M.V. -

Strike at the Mississippian Carbonate Factory
Strike at the Mississippian Carbonate Factory: Sequence Boundaries within the Mississippian Carbonates of Southeast Saskatchewan J.H.Lake, Lake Geological Services Inc, Swift Current, Sask. S9H 3W8 The depositional cyclicity of Mississippian Carbonates in the Williston Basin is well documented. Major Sequence Boundaries attests to near complete drainage of the basin (ie. top of Alida Beds / Kisbey Sands interval). Sea level drops of shorter duration also record repeated devastating shutdowns of the Carbonate Factory with resulting exposure and erosion. Sea level fluctuation patterns are repeated throughout the Alida, Frobisher and Midale Bed cycles. Repetitions include the Transgressive Surfaces of Erosion at the base of Alida (Handsworth Pool example) and base of Lower Midale (Weyburn Pool example). Peritidal muddy sediments were exposed and eroded and subsequently flooded and onlapped by higher energy sediments in both cycles. A major Sequence Boundary separates the Lower and Upper Midale ( Midale Vuggy- Midale Marly) section. This boundary preserves erosional relief in the order of 10 meters at Weyburn Pool and the total loss of the Lower Midale section to the east at Benson Pool. The Upper Midale section represents a Highstand Systems Tract with potential to preserve low energy shallow aeolian-derived clay-rich and organic-rich sediments (similar to modern Belize coastal deposits). These sediments were prone to dolomitization resulting from restricted circulation and fresh water mixing (Midale Marly reservoir). Vuggy porosity distribution throughout the section further emphasizes repeated shutdowns of the Carbonate Factory through time. The Frobisher Beds example from Hastings Pool demonstrates that vuggy porosity developed contemporaneously with sedimentation. Ripup clasts with vuggy porosity are eroded and incorporated in the overlying sediments. -

Abandoned Rail Lines in Saskatchewan
N ABANDONED RAIL LINES W E Meadow Lake IN SASKATCHEWAN S Big River Chitek Lake AS OF SEPTEMBER 2008 Frenchman Butte St. Walburg Leoville Paradise Hill Spruce Lake Debden Paddockwood Smeaton Choiceland Turtleford White Fox LLYODMINISTER Mervin Glaslyn Spiritwood Meath Park Canwood Nipawin In-Service: rail line that is still in service with a Class 1 or short- Shell Lake Medstead Marshall PRINCE ALBERT line railroad company, and for which no notice of intent to Edam Carrot River Lashburn discontinue has been entered on the railroad’s 3-year plan. Rabbit Lake Shellbrooke Maidstone Vawn Aylsham Lone Rock Parkside Gronlid Arborfield Paynton Ridgedale Meota Leask Zenon Park Macdowell Weldon To Be Discontinued: rail line currently in-service but for which Prince Birch Hills Neilburg Delmas Marcelin Hagen a notice of intent to discontinue has been entered in the railroad’s St. Louis Prairie River Erwood Star City NORTH BATTLEFORD Hoey Crooked River Hudson Bay current published 3-year plan. Krydor Blaine Lake Duck Lake Tisdale Domremy Crystal Springs MELFORT Cutknife Battleford Tway Bjorkdale Rockhaven Hafford Yellow Creek Speers Laird Sylvania Richard Pathlow Clemenceau Denholm Rosthern Recent Discontinuance: rail line which has been discontinued Rudell Wakaw St. Brieux Waldheim Porcupine Plain Maymont Pleasantdale Weekes within the past 3 years (2006 - 2008). Senlac St. Benedict Adanac Hepburn Hague Unity Radisson Cudworth Lac Vert Evesham Wilkie Middle Lake Macklin Neuanlage Archerwill Borden Naicam Cando Pilger Scott Lake Lenore Abandoned: rail line which has been discontinued / abandoned Primate Osler Reward Dalmeny Prud’homme Denzil Langham Spalding longer than 3 years ago. Note that in some cases the lines were Arelee Warman Vonda Bruno Rose Valley Salvador Usherville Landis Humbolt abandoned decades ago; rail beds may no longer be intact. -

Health Care Services Guide
423 Health Care Services Guide Sun Country Health Region TABLE OF CONTENTS SERVING THE MUNICIPALITIES AND SURROUNDING COMMUNITIES OF..... Health Region Office ................................424 Alameda Fife Lake Kisbey Pangman General Inquiries ......................................424 Ambulance ................................................424 Alida Fillmore Lake Alma Radville Bill Payments ...........................................424 Antler Forget Lampman Redvers Communications ......................................424 Arcola Frobisher Lang Roche Percee Employment Opportunities .....................424 Bengough Gainsborough Macoun Storthoaks Quality of Care Coordinator ....................424 Bienfait Gladmar Manor Stoughton Health Care Services Guide 24 Hour Help/Information Lines ..............424 Carievale Glen Ewen Maryfield Torquay Health Care Services ................................424 Carlyle Glenavon McTaggart Tribune Home Care .................................................425 Carnduff Goodwater Midale Wawota Health Care Facilities ...............................425 Ceylon Halbrite Minton Weyburn District Hospitals ......................................425 Coronach Heward North Portal Windthorst Community Hospitals ...............................425 Creelman Kennedy Ogema Yellow Grass Health Centres ..........................................425 Estevan Kenosee Lake Osage Long Term Care Facilities ........................426 Fairlight Kipling Oxbow HealthLine .................................................426 Telehealth -
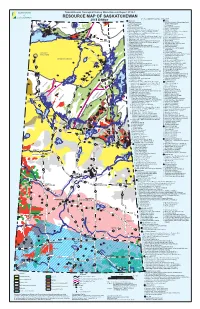
Mineral Resource Map of Saskatchewan
Saskatchewan Geological Survey Miscellaneous Report 2018-1 RESOURCE MAP OF SASKATCHEWAN KEY TO NUMBERED MINERAL DEPOSITS† 2018 Edition # URANIUM # GOLD NOLAN # # 1. Laird Island prospect 1. Box mine (closed), Athona deposit and Tazin Lake 1 Scott 4 2. Nesbitt Lake prospect Frontier Adit prospect # 2 Lake 3. 2. ELA prospect TALTSON 1 # Arty Lake deposit 2# 4. Pitch-ore mine (closed) 3. Pine Channel prospects # #3 3 TRAIN ZEMLAK 1 7 6 # DODGE ENNADAI 5. Beta Gamma mine (closed) 4. Nirdac Creek prospect 5# # #2 4# # # 8 4# 6. Eldorado HAB mine (closed) and Baska prospect 5. Ithingo Lake deposit # # # 9 BEAVERLODGE 7. 6. Twin Zone and Wedge Lake deposits URANIUM 11 # # # 6 Eldorado Eagle mine (closed) and ABC deposit CITY 13 #19# 8. National Explorations and Eldorado Dubyna mines 7. Golden Heart deposit # 15# 12 ### # 5 22 18 16 # TANTATO # (closed) and Strike deposit 8. EP and Komis mines (closed) 14 1 20 #23 # 10 1 4# 24 # 9. Eldorado Verna, Ace-Fay, Nesbitt Labine (Eagle-Ace) 9. Corner Lake deposit 2 # 5 26 # 10. Tower East and Memorial deposits 17 # ###3 # 25 and Beaverlodge mines and Bolger open pit (closed) Lake Athabasca 21 3 2 10. Martin Lake mine (closed) 11. Birch Crossing deposits Fond du Lac # Black STONY Lake 11. Rix-Athabasca, Smitty, Leonard, Cinch and Cayzor 12. Jojay deposit RAPIDS MUDJATIK Athabasca mines (closed); St. Michael prospect 13. Star Lake mine (closed) # 27 53 12. Lorado mine (closed) 14. Jolu and Decade mines (closed) 13. Black Bay/Murmac Bay mine (closed) 15. Jasper mine (closed) Fond du Lac River 14. -

16-03-06-13W2 SPILL 20140703.Pdf
October 6, 2014 Matrix 19845-523 Ms. RaeLynn Spencer CENOVUS ENERGY INC. 500 Centre Street SE Calgary, Alberta T2P 0M5 Subject: 16-03-006-13 W2M, July 3, 2014 Spill, 90-day Report Dear Mr. Harrison: 1 INTRODUCTION On July 3, 2014, Cenovus Energy Inc. commissioned Matrix Solutions Inc. to provide assistance with a produced water spill at the surface location 16-03-006-13 W2M. The spill occurred on July 3, 2014, and was caused by flowline failure originating at 01/05-24-006-14 W2M and terminating at the 01/08-23-006-14 W2M remote header. This report summarizes initial spill release information, field activities, and soil sample results. 1.1 Program Summary Results of remediation activities associated with the emulsion spill are: • The spill event involved the release of 34 m3 of emulsion. • The total spill area measured 997 m2 (972 m2 within lease boundaries and 25 m2 outside lease boundaries). • A total of 851.3 tonnes of soil and 69 m3 of fluid were removed from the spill area and transported offsite for disposal. • Soil analytical results demonstrated: electrical conductivity (EC) levels and sodium adsorption ratio (SAR) values range from below to above applicable guidelines petroleum hydrocarbons (PHC) including benzene, toluene, ethylbenzene, and xylenes (BTEX) concentrations below applicable guidelines total extractable hydrocarbon (TEH) C11-C22 and C23-C60 concentrations below applicable guidelines 1780 Railway Avenue Phone: 306.842.3088 Fax: 306.842.3356 Weyburn, Saskatchewan, Canada S4H 2K1 www.matrix-solutions.com 19845-523 20140703 LR 2014-10-06 final 2 BACKGROUND Site and spill information is summarized below in Table A.