A Review of the Welfare of Zoo Elephants in Europe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
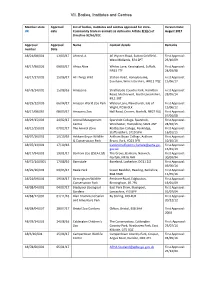
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -
Delivering a Sustainable International Visitors
Whitsun Campaign Delivering a Sustainable International Visitor Attraction…….a long road, with many successes and the odd bump! Creating Sustainable Tourism Destinations Ray Morrison, University of Chester Facilities & Environment Manager 9th July 2012 Chester Zoo Chester Zoo.......... Who are we? Our mission? Do we deliver? Do we operate the business sustainably? Key sustainability issues? Key achievements? Questions and Answers (maybe) Coffee and (maybe?) Who are we? Formed in 1934 Registered charity, conservation and education UK’s No. 1 Wildlife Attraction Top 15 zoos in World (Forbes Magazine) 1.4 million visitors in 2011 8,000 Animals, 400 different species 1,000 of Plants many endangered or rare Turnover £28 million per annum £1 million invested annually in conservation projects worldwide • Vision and Mission? Our Vision - A diverse, thriving and sustainable natural world Our Mission - To be a major force in conserving biodiversity worldwide Strategic Objective - To manage our work and activities to ensure long-term sustainability. Diverse and complex business Insight into our animal, plant, educational and environmental activities Do we deliver? ‘Sustainable’ …. Satisfying the needs of today without compromising tomorrow • Do we deliver on our mission? Evidence – Sustained achievements in line with our animal and plant conservation and educational goals, local and national. • Do we manage the Zoo’s operations sustainably? Evidence – ISO 14001, continual improvement, achieved various local and national awards Delivering -

ATIC0943 {By Email}
Animal and Plant Health Agency T 0208 2257636 Access to Information Team F 01932 357608 Weybourne Building Ground Floor Woodham Lane www.gov.uk/apha New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC0943 {By Email} 4 October 2016 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about zoos which we received on 26 September 2016. Your request has been handled under the Freedom of Information Act 2000. The information you requested and our response is detailed below: “Please can you provide me with a full list of the names of all Zoos in the UK. Under the classification of 'Zoos' I am including any place where a member of the public can visit or observe captive animals: zoological parks, centres or gardens; aquariums, oceanariums or aquatic attractions; wildlife centres; butterfly farms; petting farms or petting zoos. “Please also provide me the date of when each zoo has received its license under the Zoo License act 1981.” See Appendix 1 for a list that APHA hold on current licensed zoos affected by the Zoo License Act 1981 in Great Britain (England, Scotland and Wales), as at 26 September 2016 (date of request). The information relating to Northern Ireland is not held by APHA. Any potential information maybe held with the Department of Agriculture, Environment and Rural Affairs Northern Ireland (DAERA-NI). Where there are blanks on the zoo license start date that means the information you have requested is not held by APHA. Please note that the Local Authorities’ Trading Standard departments are responsible for administering and issuing zoo licensing under the Zoo Licensing Act 1981. -

A Stella Year the Park Is Celebrating Its 50Th Anniversary in 2020
2020 WILD TALKwww.cotswoldwildlifepark.co.uk New baby White Rhino Stella cuddles up to her mum Ruby Photo: Rory Carnegie Rory Photo: A Stella Year The Park is celebrating its 50th Anniversary in 2020. We hope to continue to inspire future Ruby and Stella walked out of the stall, giving generations to appreciate the beauty of the lucky visitors a glimpse of a baby White Rhino natural world. 2019 was a great year with Soon after the birth, Ruby and her new calf record visitor numbers, TV appearances, lots walked out of the stall into the sunshine of the of baby animals and a new Rhino calf. yard, giving a few lucky visitors a glimpse of a baby White Rhino less than two hours old. ighlights of the year included the Stella is doing well and Ruby has proved once Park featuring on BBC’s Springwatch again to be an exceptional mother. Having Hprogramme, with our part in the White another female calf is really important for the Storks’ UK re-introduction project. Then in European Breeding Programme of this iconic Astrid October, BBC Gardeners’ World featured the but endangered species. Park and presenter Adam Frost sang the praises OUR 6 RHINO CALVES of our gardens. To top off the year, Ruby gave White Rhinos have always been an important 1. Astrid born 1st July 2013, birth to our sixth Rhino calf in as many years, species at the Park, which was founded by John moved to Colchester Zoo. named “Stella”! Heyworth (1925-2012) in 1970. He had a soft spot 2. -

Elephant Ivory, Zoos, and Extinction in the Age of Imperialism
East and West This enduring fascination with elephants turned their bodies into colonial trophies, dead or alive. While captive elephants were the hallmarks of European zoological collections, ivory RESEARCH TOPICS became a luxurious commodity and an expression of wealth. At the turn of the twentieth century, patterns of ivory consumption were closely connected to the growing middle-class in Europe and the United States. As a status symbol ivory signaled elevation in social rank, N°63 especially for those whose belonging to European culture or even whiteness was in doubt. Thus, recording consumer desire for ivory in Eastern Europe, a region that, on the one hand, largely “missed out” on securing its own African or Asian colonies, but one that nevertheless nurtured colonial longings, reveals close links between social constructions of race, colonial commerce, and animal bodies. For the re- gion caught between the constructions of “progressive Elephant Ivory, Zoos, and West” and “backwards East,” the elephant body served as a material link between the mystical Orient and the Extinction in the Age of colonial Empire, and helped to measure the differenc- es of savagery and civilization. Imperialism (1870s–1940s) 04 A pair of porcelain perfume bottles placed As part of Department III’s research theme “The Body by Marianna Szczygielska on an ivory stand. First half of the 19th century. of Animals,” this project offers a unique insight into JULY 2019 Source: RDW MIC, Virtual Małopolska project. a physical presence of colonial imperialism in an area without overseas colonies. Tracing elephant lives, deaths, and afterlives, all entwined with stories of their keepers, trainers and veterinarians, uncovers a variety of scientific practices and technologies behind the exotic animal trade. -

NICK ELLERTON 5 February 1949 – 29 March 2014
NICK ELLERTON 5 February 1949 – 29 March 2014 Obituary Sadly this reports that Nick really knew and cared about cook, a red wine lover and a loyal Ellerton died suddenly in the early animal welfare and was prepared friend. At times he had some morning of Saturday 29 March to be vocal about those issues. He outrageous opinions, strongly 2014, when he suffered a heart always put the needs of the expressed, but was always willing attack, in Sri Lanka. animals first and foremost, even to listen to challenges to those though it made him politically views, even if this did not always Nick worked as Deputy, then unpopular at times. He was a man shift his own. Curator of Mammals at the North ahead of his time in Zoos, and of England Zoological Society particularly drove changes in our Nick was probably the most (Chester Zoo) for 31 years before attitude to elephant welfare and observant person I have ever moving to Knowsley Safari Park for breeding so forcefully back in the known. He noticed everything. 11 years. Together with his 1980s. He was able to get a lot of He felt strongly about looking after longtime partner Penny Boyd progressive ideas around animal his own back yard before (formerly of Burstow Wildlife welfare started and worried little suggesting to other countries how Sanctuary in the UK, and latterly about the fallout for those who to look after theirs. He was also at Knowsley) they moved to were not prepared to change their realistic in the advice he offered to Sri Lanka in the summer of 2010 attitudes. -

Visitor Attraction Trends England 2003 Presents the Findings of the Survey of Visits to Visitor Attractions Undertaken in England by Visitbritain
Visitor Attraction Trends England 2003 ACKNOWLEDGEMENTS VisitBritain would like to thank all representatives and operators in the attraction sector who provided information for the national survey on which this report is based. No part of this publication may be reproduced for commercial purp oses without previous written consent of VisitBritain. Extracts may be quoted if the source is acknowledged. Statistics in this report are given in good faith on the basis of information provided by proprietors of attractions. VisitBritain regrets it can not guarantee the accuracy of the information contained in this report nor accept responsibility for error or misrepresentation. Published by VisitBritain (incorporated under the 1969 Development of Tourism Act as the British Tourist Authority) © 2004 Bri tish Tourist Authority (trading as VisitBritain) Cover images © www.britainonview.com From left to right: Alnwick Castle, Legoland Windsor, Kent and East Sussex Railway, Royal Academy of Arts, Penshurst Place VisitBritain is grateful to English Heritage and the MLA for their financial support for the 2003 survey. ISBN 0 7095 8022 3 September 2004 VISITOR ATTR ACTION TRENDS ENGLAND 2003 2 CONTENTS CONTENTS A KEY FINDINGS 4 1 INTRODUCTION AND BACKGROUND 12 1.1 Research objectives 12 1.2 Survey method 13 1.3 Population, sample and response rate 13 1.4 Guide to the tables 15 2 ENGLAND VISIT TRENDS 2002 -2003 17 2.1 England visit trends 2002 -2003 by attraction category 17 2.2 England visit trends 2002 -2003 by admission type 18 2.3 England visit trends -

The International Elephant Foundation Strategy In
INTERNATIONAL ELEPHANT FOUNDATION STRATEGY IN SUPPORT OF ASIAN ELEPHANT CONSERVATION The International Elephant Foundation Strategy in Support of Asian Elephant Conservation is the result of the International Elephant Foundation (IEF) facilitated workshop of technical representatives from U.S. Asian elephant facilities with expertise conserving Asian elephants in human care, and other U.S. representatives with expertise and experience conserving Asian elephants in range countries. The goal of this Action Plan is to enhance and conserve Asian elephant populations in the wild. Mission Statement The International Elephant Foundation Strategy in Support of Asian Elephant Conservation provides a more coordinated Asian elephant conservation strategy for U.S. Asian elephant facilities focusing on the expertise and experience of the U.S. elephant management community. Vision Statement This strategy identifies and describes those specific components of in situ Asian elephant conservation where there is a direct link to ex situ Asian elephant expertise, and identifies suggested management actions. With a priority focused list of actions, the U.S. elephant management community can maximize limited resources, encourage coordination and collaboration, and further encourage increased participation resulting in a more coordinated approach to maximize conservation activities. 1. Background Asian elephants were historically found from West Asia along the Iranian coast into the Indian subcontinent, and eastward into Southeast Asia and parts of China. Formerly ranging over three and a half million square miles, the Asian elephant is now extinct in West Asia, Java, and most of China, and survives in isolated populations scattered across remaining grassland and tropical forest habitats in thirteen Asian countries. Less than 30% of the entire extant range is within protected areas, and many protected areas afford little protection for elephants or their habitat. -

Ks2 Sumatran Tiger Answers (Virtual Zoo Day)
LEARN AT KS2 SUMATRAN TIGER QUESTIONS (VIRTUAL ZOO DAY) After watching our Sumatran tigers as part of our Virtual Zoo Day, test your knowledge with the following questions. Write your answers as full sentences. QUESTION 1 What do tigers use their stripes for? .............................................................................................................................................................................................................................................. QUESTION 2 We have two Sumatran tigers, a mother and daughter, what are their names? ............................................................................................................................................................................................................................................. QUESTION 3 Orangutan Which special teeth help tigers to sheer through meat? .............................................................................................................................................................................................................................................. QUESTION 4 Sumatran tigers are the smallest subspecies of tiger but how many subspecies of tiger can be found in the world today? ............................................................................................................................................................................................................................................. QUESTION 5 Sumatran tigers are critically endangered, can -

Concepts in the Care and Welfare of Captive Elephants J
REVIEW: ELEPHANTS CONCEPTS IN CARE AND WELFARE IN CAPTIVITY 63 Int. Zoo Yb. (2006) 40: 63–79 © The Zoological Society of London Concepts in the care and welfare of captive elephants J. VEASEY Woburn Safari Park, Woburn, Bedfordshire MK17 9QN, United Kingdom E-mail: [email protected] Zoos are duty bound to maintain a high standard of challenges facing zoos both from an welfare for all animals for which they are respons- ible. For elephants, this represents a greater chal- animal husbandry and a public perception lenge than for many other species; their sheer size, perspective. This document is not sophisticated social life, high level of intelligence and designed to be a formula for elephant wel- large behavioural repertoire, combined with their fare in captivity because there are still too origins in tropical and subtropical climates mean that replicating the physical, social and environ- many un-answered questions, rather it is mental requirements needed for a high standard of intended as a discussion of some of the welfare in captivity is a significant challenge. This is concepts relating to captive elephant wel- compounded by the difficulties in measuring welfare generally, and specifically for animals such as ele- fare, which it is hoped, might encourage phants within zoo environments. Evidence does exist people from a variety of viewpoints to relating to the longevity, reproductive success and consider elephant welfare in a different the health status of captive elephants which suggests way. I will not go into the issue of whether that their management is not at as high a standard as it is for many other species kept in zoos, and that or not elephants should be in captivity elephant welfare is likely to be compromised as a because this would be a not inconsider- result. -

The Release of a Captive-Raised Female African Elephant (Loxodonta Africana) in the Okavango Delta, Botswana
Animals 2013, 3, 370-385; doi:10.3390/ani3020370 OPEN ACCESS animals ISSN 2076-2615 www.mdpi.com/journal/animals Article The Release of a Captive-Raised Female African Elephant (Loxodonta africana) in the Okavango Delta, Botswana Kate Evans 1,2,*, Randall J. Moore 3 and Stephen Harris 1 1 School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, UK; E-Mail: [email protected] 2 Elephants For Africa, P.O. Box HA148 HAK, Maun, Botswana 3 Elephant Back Safaris, Private Bag 332, Maun, Botswana; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +44-117-928-7479; Fax: +44-117-331-7985. Received: 28 March 2013; in revised form: 22 April 2013 / Accepted: 22 April 2013 / Published: 29 April 2013 Simple Summary: Managing captive elephants poses a significant challenge because of their complex social behaviour. While wild female elephants live in close-knit family groups of related individuals, captive herds often consist of unrelated animals. Some of the elephants in captive groups may be excluded by their companions and experience increased aggression, so that their welfare is compromised. There is no easy solution to this problem and novel approaches are required since slaughter of captive elephants is not publicly acceptable. We show that captive-raised female elephants can be released into the wild, survive and reproduce, and suggest that this management option should be explored further for female elephants currently held under various captive conditions. Abstract: Wild female elephants live in close-knit matrilineal groups and housing captive elephants in artificial social groupings can cause significant welfare issues for individuals not accepted by other group members. -

The Ethnography of Captive Elephant Management in Nepal: a Synopsis
Gajah 34 (2011) 32-40 The Ethnography of Captive Elephant Management in Nepal: A Synopsis Piers Locke University of Canterbury, Christchurch, New Zealand Author’s e-mail: [email protected] Introduction Nepal may not have seemed like the most obvious choice of location for this ethnographic kind of In 2001, with the help of my research assistant Satya research, since its captive elephant management Man Lama, I embarked upon an anthropological practices are not nearly as well known as those study of captive elephant management in of India, Sri Lanka, Myanmar, and Thailand, and Nepal. This has been a long-term programme perhaps also since the number of captive elephants of ethnographic research entailing participant is so small (236 registered as of 2011). However, observation, semi-structured interviewing, the presence of the government elephant stable, survey data, photographic documentation, or sarkari hattisar, as an integral component historical investigation, and textual translation. of the national parks and wildlife reserves of My doctoral fieldwork additionally involved my Nepal’s lowland Tarai region, and the Khorsor own apprenticeship as an elephant handler at the Elephant Breeding Center at which captive born Khorsor Elephant Breeding Center in Chitwan, elephants are trained, made Nepal a particularly where the handlers explained that I could never attractive field site. Since 2001 I have made four understand their life and work unless I experienced successive research trips totalling 20 months of it for myself. Khorsor is also the site at which we fieldwork. In what follows, I introduce the world shot the documentary film Servants of Ganesh, of Nepali captive elephant management and which concerns the training of a juvenile elephant briefly summarize a few of the key issues I have called Paras Gaj (Dugas & Locke 2007).