Sheela Minor Project.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problems of Salination of Land in Coastal Areas of India and Suitable Protection Measures
Government of India Ministry of Water Resources, River Development & Ganga Rejuvenation A report on Problems of Salination of Land in Coastal Areas of India and Suitable Protection Measures Hydrological Studies Organization Central Water Commission New Delhi July, 2017 'qffif ~ "1~~ cg'il'( ~ \jf"(>f 3mft1T Narendra Kumar \jf"(>f -«mur~' ;:rcft fctq;m 3tR 1'j1n WefOT q?II cl<l 3re2iM q;a:m ~0 315 ('G),~ '1cA ~ ~ tf~q, 1{ffit tf'(Chl '( 3TR. cfi. ~. ~ ~-110066 Chairman Government of India Central Water Commission & Ex-Officio Secretary to the Govt. of India Ministry of Water Resources, River Development and Ganga Rejuvenation Room No. 315 (S), Sewa Bhawan R. K. Puram, New Delhi-110066 FOREWORD Salinity is a significant challenge and poses risks to sustainable development of Coastal regions of India. If left unmanaged, salinity has serious implications for water quality, biodiversity, agricultural productivity, supply of water for critical human needs and industry and the longevity of infrastructure. The Coastal Salinity has become a persistent problem due to ingress of the sea water inland. This is the most significant environmental and economical challenge and needs immediate attention. The coastal areas are more susceptible as these are pockets of development in the country. Most of the trade happens in the coastal areas which lead to extensive migration in the coastal areas. This led to the depletion of the coastal fresh water resources. Digging more and more deeper wells has led to the ingress of sea water into the fresh water aquifers turning them saline. The rainfall patterns, water resources, geology/hydro-geology vary from region to region along the coastal belt. -

List of Teachers Posted from the Following Schools to Various Examination Centers As Assistant Superintendents for Higher Secondary Exam March 2015
LIST OF TEACHERS POSTED FROM THE FOLLOWING SCHOOLS TO VARIOUS EXAMINATION CENTERS AS ASSISTANT SUPERINTENDENTS FOR HIGHER SECONDARY EXAM MARCH 2015 08001 - GOVT SMT HSS,CHELAKKARA,THRISSUR 1 DILEEP KUMAR P V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04884231495, 9495222963 2 SWAPNA P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9846374117 3 SHAHINA.K 08035-GOVT. RSR VHSS, VELUR, THRISSUR 04885241085, 9447751409 4 SEENA M 08041-GOVT HSS,PAZHAYANNOOR,THRISSUR 04884254389, 9447674312 5 SEENA P.R 08046-AKM HSS,POOCHATTY,THRISSUR 04872356188, 9947088692 6 BINDHU C 08062-ST ANTONY S HSS,PUDUKAD,THRISSUR 04842331819, 9961991555 7 SINDHU K 08137-GOVT. MODEL HSS FOR GIRLS, THRISSUR TOWN, , 9037873800 THRISSUR 8 SREEDEVI.S 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9020409594 9 RADHIKA.R 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742552608, 9847122431 10 VINOD P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9446146634 11 LATHIKADEVI L A 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742482838, 9048923857 12 REJEESH KUMAR.V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04762831245, 9447986101 08002 - GOVT HSS,CHERPU,THRISSUR 1 PREETHY M K 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR 04802820505, 9496288495 2 RADHIKA C S 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR , 9495853650 3 THRESSIA A.O 08005-GOVT HSS,KODAKARA,THRISSUR 04802726280, 9048784499 4 SMITHA M.K 08046-AKM HSS,POOCHATTY,THRISSUR 04872317979, 8547619054 5 RADHA M.R 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872342425, 9497180518 6 JANITHA K 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872448686, 9744670871 1 7 SREELEKHA.E.S 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872343515, 9446541276 8 APINDAS T T 08095-ST. PAULS CONVENT EHSS KURIACHIRA, THRISSUR, 04872342644, 9446627146 680006 9 M.JAMILA BEEVI 08107-SN GHSS, KANIMANGALAM, THRISSUR, 680027 , 9388553667 10 MANJULA V R 08118-TECHNICAL HSS, VARADIAM, THRISSUR, 680547 04872216227, 9446417919 11 BETSY C V 08138-GOVT. -
List of Lacs with Local Body Segments (PDF
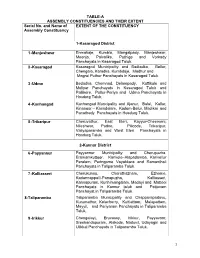
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -

Working Group Report- Fisheries 13Th Five
GOVERNMENT OF KERALA STATE PLANNING BOARD THIRTEENTH FIVE-YEAR PLAN 2017-2022 WORKING GROUP ON FISHERIES REPORT AGRICULTURE DIVISION THIRUVANANTHAPURAM MARCH 2017 PREFACE In Kerala, the process of a Five-Year Plan is an exercise in people’s participation. At the end of September 2016, the Kerala State Planning Board began an effort to conduct the widest possible consultations before formulating the Plan. The Planning Board formed 43 Working Groups, with a total of more than 700 members – scholars, administrators, social and political activists and other experts. Although the Reports do not represent the official position of the Government of Kerala, their content will help in the formulation of the Thirteenth Five-Year Plan document. This document is the report of the Working Group on Fisheries. The Chairpersons of the Working Group were Shri P James Varghese IAS and Dr C Mohanakumaran Nair. The Member of the Planning Board who coordinated the activities of the Working Group was Professor R Ramakumar. The concerned Chief of Division was Dr P Rajasekharan. Member Secretary CONTENTS Chapter 1 Introduction ............................................................................................................................................... 1 Chapter 2 Review of 12th Five-Year Plan Schemes ............................................................................................... 4 Overview of Finances ............................................................................................................................................ -

Accused Persons Arrested in Thrissur Rural District from 03.09.2017 to 09.09.2017
Accused Persons arrested in Thrissur Rural district from 03.09.2017 to 09.09.2017 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 KANAKKASSERY CR.40/17 U/S HOUSE 03.09.201 KRISHNAN 34/17 KALLETTUMK 15 (C ) R/W VIMAL JFCM 1 KANNAN THAZHEKKAD 7 AT 18.45 ALOOR KUTTY MALE ARA 63 OF ABKARI SI OF POLICE CHALAKUDY DESAM THAZHAKAD HRS ACT VILLAGE THRISSUR CR.1123/17 03.09.201 56/17 KUTTIPARAMBAN(H CHALAKUDY U/S 15 (C ) JAYESHBALAN JFCM 2 MOHANAN KUNJAN 7 AT 18.25 CHALAKUDY MALE ),POTTA THRISSUR SOUTH R/W 63 OF SI OF POLICE CHALAKUDY HRS ABKARI ACT PANIKKAVEETIL CR.705/17 SHINU 03.09.201 JFCM SHAHUL 22/17 HOUSE,KATTOOR U/S 341 323 AJESH 3 SHAHUL KATTOOR 7 AT 13.54 KATTOOR IRINJALAKUD HAMEED MALE SNDP, KATTOOR 324 506 (I) SI OF POLICE HAMMED HRS A VILLAGE THRISSUR R/W 34 IPC IRINGATHURUTHI 03.09.201 CR.734/17 MANSHOOR JFCM 52/17 HOUSE ,PADIYOOR, 4 MANI KUNJAPPAN KATTOOR 7 AT 12.48 U/S 341 323 KATTOOR ADDL SI OF IRINJALAKUD MALE PADIYOOR, HRS R/W 34 IPC POLICE A THRISSUR MURUKKUMTHARA HOUSE, 03.09.201 CR.734/17 MANSHOOR JFCM NARAYANKU 35/17 5 SIBIN KOORIKUZHY KATTOOR 7 AT 12.57 U/S 341 323 KATTOOR ADDL SI OF IRINJALAKUD TTY MALE ,KAIPAMANGALAM HRS R/W 34 IPC POLICE A THRISSUR IRINGATHURUTHI HOUSE 03.09.201 CR.734/17 MANSHOOR JFCM 56/17 6 SIDHARTHAN KUNJAPPAN ,KOORIKUZHI, KATTOOR 7 AT 12.59 U/S 341 323 KATTOOR ADDL SI -

Report of Rapid Impact Assessment of Flood/ Landslides on Biodiversity Focus on Community Perspectives of the Affect on Biodiversity and Ecosystems
IMPACT OF FLOOD/ LANDSLIDES ON BIODIVERSITY COMMUNITY PERSPECTIVES AUGUST 2018 KERALA state BIODIVERSITY board 1 IMPACT OF FLOOD/LANDSLIDES ON BIODIVERSITY - COMMUnity Perspectives August 2018 Editor in Chief Dr S.C. Joshi IFS (Retd) Chairman, Kerala State Biodiversity Board, Thiruvananthapuram Editorial team Dr. V. Balakrishnan Member Secretary, Kerala State Biodiversity Board Dr. Preetha N. Mrs. Mithrambika N. B. Dr. Baiju Lal B. Dr .Pradeep S. Dr . Suresh T. Mrs. Sunitha Menon Typography : Mrs. Ajmi U.R. Design: Shinelal Published by Kerala State Biodiversity Board, Thiruvananthapuram 2 FOREWORD Kerala is the only state in India where Biodiversity Management Committees (BMC) has been constituted in all Panchayats, Municipalities and Corporation way back in 2012. The BMCs of Kerala has also been declared as Environmental watch groups by the Government of Kerala vide GO No 04/13/Envt dated 13.05.2013. In Kerala after the devastating natural disasters of August 2018 Post Disaster Needs Assessment ( PDNA) has been conducted officially by international organizations. The present report of Rapid Impact Assessment of flood/ landslides on Biodiversity focus on community perspectives of the affect on Biodiversity and Ecosystems. It is for the first time in India that such an assessment of impact of natural disasters on Biodiversity was conducted at LSG level and it is a collaborative effort of BMC and Kerala State Biodiversity Board (KSBB). More importantly each of the 187 BMCs who were involved had also outlined the major causes for such an impact as perceived by them and suggested strategies for biodiversity conservation at local level. Being a study conducted by local community all efforts has been made to incorporate practical approaches for prioritizing areas for biodiversity conservation which can be implemented at local level. -

Accused Persons Arrested in Thrissur Rural District from 29.11.2020To05.12.2020
Accused Persons arrested in Thrissur Rural district from 29.11.2020to05.12.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 29-11-2020 1050/2020 CHERPU UNNIKRISH MOHANDA 50, URAKATH HOUSE PERINCHER BAILED BY 1 APPU at 21:35 U/s 118(e) of (Thrissur NAN S Male , PERINCHERY RY POLICE Hrs KP Act Rural) SI OF POLICE 1281/2020 U/s 269, 270 IPC & 118(e) of KP Act & ANANDAKR MATHILAK POOVALIPARAMB 29-11-2020 Sec. 4(2)(f) ISHNAN SHAMSUDE 56, AM BAILED BY 2 ABDU IL HOUSE, AMANDOOR at 20:30 r/w 5 of ISHO EN Male (Thrissur POLICE PORIBAZAR Hrs Kerala MATHILAKA Rural) Epidemic M PS Diseases Ordinance 2020 THEKKOTTU 991/2020 U/s HOUSE, PARAMBI 29-11-2020 ALOOR SUKUMARA 35, PARAMBI 4(2)(e)(j)r/w SREEJITH.AK BAILED BY 3 ARUN ROAD DESAM, at 20:50 (Thrissur N Male ROAD 3(a) of KEDO SI OF POLICE POLICE THAZHEKKAD Hrs Rural) 2020 VILLAGE THEKKOTU 991/2020 U/s HOUSE,THAZHEK 29-11-2020 ALOOR 38, PARAMBI 4(2)(e)(j)r/w SREEJITH.AK BAILED BY 4 SAJI MOHANAN AD at 20:50 (Thrissur Male ROAD 3(a) of KEDO SI OF POLICE POLICE DESHAM,THAZHE Hrs Rural) 2020 KAD VILLAGE 1280/2020 U/s 269, 270 IPC & 118(e) RAMANKULATH of KP Act & MATHILAK HOUSE, EMMAD 29-11-2020 Sec. -
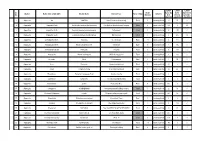
Sl. No. District Name of the LSGD (CDS)
LUNCH LUNCH LUNCH Sl. No Of Parcel By Home Sponsored District Name of the LSGD (CDS) Kitchen Name Kitchen Place Rural / Urban Initiative No. Members Unit Delivery by LSGI's (April 23) (April 23) (April 23) 1 Alappuzha Ala JANATHA Near CSI church, Kodukulanji Rural 5 Janakeeya Hotel 10 65 0 2 Alappuzha Alappuzha North Ruchikoottu Janakiya Bhakshanasala Coir Machine Manufacturing Company Urban 4 Janakeeya Hotel 122 0 20 3 Alappuzha Alappuzha South Samrudhi janakeeya bhakshanashal Pazhaveedu Urban 5 Janakeeya Hotel 80 155 0 4 Alappuzha Alappuzha South Community kitchen thavakkal group MCH junction Urban 5 Janakeeya Hotel 0 355 12 5 Alappuzha Ambalppuzha North Swaruma Neerkkunnam Rural 10 Janakeeya Hotel 0 100 2 6 Alappuzha Ambalppuzha North Annam Janakeeya Hotel Vandanam Rural 7 Janakeeya Hotel 0 152 0 7 Alappuzha Ambalappuzha South Patheyam Amayida Rural 5 Janakeeya Hotel 0 166 6 8 Alappuzha Arattupuzha Hanna catering unit JMS hall,arattupuzha Rural 6 Janakeeya Hotel 8 132 0 9 Alappuzha Arookutty Ruchi Kombanamuri Rural 5 Janakeeya Hotel 7 96 0 10 Alappuzha Aroor Navaruchi Vyasa charitable trust Rural 5 Janakeeya Hotel 40 0 0 11 Alappuzha Aryad Anagha Catering Near Aryad Panchayat Rural 5 Janakeeya Hotel 74 40 0 12 Alappuzha Bharanikavu Sasneham Janakeeya Hotel Koyickal chantha Rural 5 Janakeeya Hotel 0 0 0 13 Alappuzha Budhanoor sampoorna mooshari parampil building Rural 5 Janakeeya Hotel 0 0 0 14 Alappuzha Chambakulam Jyothis Near party office Rural 4 Janakeeya Hotel 193 0 0 15 Alappuzha Chenganoor SRAMADANAM chengannur market building -

National Huda Central School, Orumanayur
NATIONAL HUDA CENTRAL SCHOOL, ORUMANAYUR Information of the school as per the direction of CBSE 1. Name of the School with address: NATIONAL HUDA CENTRAL SCHOOL, ORUMANAYUR, THRISSUR, KERALA – 680 512 (i) E-mail : [email protected], [email protected] (ii) Ph. No. : 0487 2502037, 2503759, 8281553972 (iii) Fax No. : NIL 2. Year of establishment of the school : 1995 3. Whether NOC from State/UTorrecommendationof Embassy of India obtained? : NOC obtained from the Gov. of Kerala. (i) NOC No. : 5410/N3/2001/G.Edn. (ii) NOC issuing date: 30.03.2001 4. Is the school recognised, if yesby which authority : Affiliated to Central Board of Secondary Education,New Delhi 5. Status of affiliation: Provisional (i) Affiliation No. 930458 (ii) Affiliation with Board since: 01.04.2004 (iii) Extension of affiliation upto: 31.03.2019 6. Name of Trust/Society/CompanyRegistered under Section 25 ofthe Company Act, 1956. : Hidayath Charitable Society (Reg. No. 769/200 dt. 08.11.2000 Period upto which Registrationof Trust/Society is valid : 8 November, 2019 7. List of members of School Managing Committee SL. NO. NAME DESIGNATION CLAUSE IN AFFILIATION BYE LAWS, CHAPTER VI SL NO. NAME DESIGNATION 1 MAMUNNY MAULAVI CHAIRMAN 2 MOHAMED AMEEN ACTING CHAIRMAN 3 A MOIDUNNY VICE CHAIRMAN 4 A T MUSTHAFA SECRETARY 5 P BACKER JOINT SECRETARY 6 OMER KOYA TREASURER 7 MOHAMMED ALI EXECUTIVE MEMBER 8 P K MOHAMMEDUNNY EXECUTIVE MEMBER 9 V P MOIDHU EXECUTIVE MEMBER 10 P K NOORUDHEEN EXECUTIVE MEMBER 11 P SAIFUDHEEN EXECUTIVE MEMBER 12 K V SHIHAB EXECUTIVE MEMBER 13 T ABOOBACKER EXECUTIVE MEMBER 14 E K ABDUL RASAK MEMBER 15 V P SHAHUL HAMEED MEMBER 16 A V KADERMON MEMBER 17 P O ALIKUTTY MEMBER 18 P V EBRAHIM MEMBER 19 A P KASMI MEMBER 20 M IBRAHIMKUTTY MEMBER SAIDHABI 21 MEMBER SAIDMOHAMMED 22 SHAMSUDHEEN K M MEMBER MUMTHAZ ABDUL 23 MEMBER KAREEM 24 FAISAL USMAN MOOPPIL MEMBER 8. -

No Name School Address Residence Address Photo 1 Ajitha K National Hss, Irinjalakuda, Ph: 0480- 2821248 Sopanam', Pattamali
NO NAME SCHOOL ADDRESS RESIDENCE ADDRESS PHOTO NATIONAL HSS, SOPANAM', PATTAMALI ROAD, 1 AJITHA K IRINJALAKUDA, PH: 0480- IRINJALAKUDA, 0480-2856444, 2821248 9400163990 ST.CLARE'S CGHSS,TCR, W/O K.M FRANKLIN, KOOLA(H), 2 ANIT P ANTONY 2334432, ANCHAGADY, P.O ARANATTUKARA, [email protected] 9446768220 ILLATHU, KALLIPADAM P.O, KULLAPULLY, GVHSS FOR DEAF, 3 ANITHA K.G SHORNUR, 9447334579, 9447334579, KUNNAMKULAM [email protected] ANITHA KNM VHSS, VATANAPPALLY, KELAMKANDATH HOUSE,ANTHIKAD, 4 MUKUNDAN 0487-2290030 0487-2631938, 8129267675 GHSS ERUMAPETTY, ARIYARATH HOUSE, KIZHOOR, 5 ANJANA C.A ERUMAPETTY,THRISSUR, KUNNAMKULAM, 04885-228366, 04885-264239 9645374280, [email protected] MES HSS, KOLLEZHUTH (H), P.O VEMBALLOOR- SREENARAYANAPURAM, PH: 6 ANJU K.K 680671, 0480-2851924, 9447970105, 0480-2854263, [email protected] [email protected] CHALDEAN SYRIAN HSS,TCR, CHIRAYATH,VAKAYIL ANNE SWAPNA 0487-2422791, ROAD,CHIYYARAM,TCR, 04872250709, 7 MATHEW chaldeansyrianhsstrichur@ya 9447437030,anneswapnamathew@gmail hoo.com .com GHSS AYYATHOLE, KARIAT D4A11KSHB, PULLAZHI P.O, AYYATHOLE P.O ,THRISSUR, 8 ARIFA K M THRISSUR, 680012, PH: 0487-2364218, 680003, PH: 0487-2363437, 9446143474, [email protected] [email protected] ERASSERY(H),EDAMUTTAM- GOVT.GIRLS HSS, 680568,THRISSUR(DT),0480- 9 BABY C.K KODUNGALLUR, 0480- 2835745,9446145598, 2812580 [email protected] MANJALY HOUSE,P.O PUDUKAD,TSR- ST.ANTONY'S 680301,0480- 10 BEBY HELEN HSS,PUDUKAD,0480-2755242 2758516,9497740001,bbmanjaly@yahoo. com HSS PANANGAD,P.O CHEEREPARAMBIL(H), -

Accused Persons Arrested in Thrissur Rural District from 11.02.2018 to 17.02.2018
Accused Persons arrested in Thrissur Rural district from 11.02.2018 to 17.02.2018 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 AINIKKAL HOUSE, 11.02.201 CR.141/18 52/18 PERINGOTTU S.R.SANEESH. JFCM NO II 1 MOHANAN AYYAPPAN KIZHUPPILLIKKARA 8 AT 16.50 U/S 15 OF KG ANTHIKAD MALE KKARA SI OF POLICE THRISSUR THRISSUR HRS ACT 11.02.201 CR.141/18 28/18 NJATTUVETTY PERINGOTTU S.R.SANEESH. JFCM NO II 2 KIRAN DASAN 8 AT 16.50 U/S 15 OF KG ANTHIKAD MALE HOUSE THRISSUR KKARA SI OF POLICE THRISSUR HRS ACT KARTTUPARAMBIL HOUSE, 11.02.201 CR.141/18 SANKARA 40/18 PERINGOTTU S.R.SANEESH. JFCM NO II 3 SUDHIR K S KIZHUPPILLIKKARA, 8 AT 16.50 U/S 15 OF KG ANTHIKAD NARAYANAN MALE KKARA SI OF POLICE THRISSUR THANNYAM HRS ACT THRISSUR CR.142/18 VALLANGAPARAMBI 11.02.201 53/18 U/S 15 (C ) S.R.SANEESH. JFCM NO II 4 SAHADEVAN AYYAPPAN L HOUSE, MANALUR MANALOOR 8 AT 17.20 ANTHIKAD MALE R/W 63 OF SI OF POLICE THRISSUR THRISSUR HRS ABAKARI ACT CR.142/18 ELVALLY HOUSE, 11.02.201 43/18 U/S 15 (C ) S.R.SANEESH. JFCM NO II 5 BABU KOCHUMON MANALUR MANALOOR 8 AT 17.20 ANTHIKAD MALE R/W 63 OF SI OF POLICE THRISSUR THRISSUR HRS ABAKARI ACT CR.143/18 ORIPARAMBIL 11.02.201 36/18 U/S 15 (C ) S.R.SANEESH. -

Jeslin Joseph K
Jeslin Joseph K Kallarakkal house Krishnankotta P.O, Poyya Thrissur District Pin: 680733 Ph : 9605074486, 9446787367 PERSONAL DETAILS Name : JESLIN JOSEPH.K Father’s Name : K.C.JOSEPH Mother’s Name : Mercy Joseph.K Age : 27 Yrs. Date of birth : 27-5-1990 Marital status : Married Permanent address : Kallarakkal House Krishanakotta .P.O Poyya Thrissur District Kerala state Pin : 680733 E-mail : [email protected] Nationality : Indian Languages Known : Malayalam, English, Hindi and Tamil Hobbies : Reading, Listening Music EDUCATIONAL QUALIFICATION Master of Optometry (2012 -2014) Lotus Bausch and Lomb Institute of Optometry Bharathiyar University, Coimbatore, Tamilnadu Mark Obtained 72% BSc.Optometry (2007 – 2011) Lotus Bausch and Lomb Institute of Optometry Allagappa university,Tamilnadu Mark Obtained 70% Higher Secondary (2005 -2007) Soccorso Convent Girls Higher Secondary School, Mala Mark obtained 81% SSLC ( 2005) St. Anne’s High School, Kottapuram Mark obtained 86% WORK EXPERIENCE • Worked as Consultant optometrist at Giridhar eye institute, Kadavanthra, Cochin from 2015 to 2016 • Practiced as consultant optometrist, ZYOPTIX In –Charge at VASAN EYE CARE HOSPITAL,COCHIN SHIPYARD BRANCH from 2011 to 2012 • Practiced as consultant optometrist and Faculty in optometry at LOTUS EYE CARE HOSPITAL AND INSTITUTE, Coimbatore ; during the period of 2012 to 2014 • 1 year internship in LOTUS EYE CARE HOSPITAL AND INSTITUTE, Tamilnadu (2010 to 2011) During my work period, I have been practiced with following departments: CORNEA CLINIC • Refraction